Cbx4 SUMOylates BRD4 to regulate the expression of inflammatory cytokines in post-traumatic osteoarthritis
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Brominated domain protein 4 (BRD4) is a chromatin reader known to exacerbate the inflammatory response in post-traumatic osteoarthritis (PTOA) by controlling the expression of inflammatory cytokines. However, the extent to which this regulatory effect is altered after BRD4 translation remains largely unknown. In this study, we showed that the E3 SUMO protein ligase CBX4 (Cbx4) is involved in the SUMO modification of BRD4 to affect its ability to control the expression of the proinflammatory genes IL-1β, TNF-α, and IL-6 in synovial fibroblasts. Specifically, Cbx4-mediated SUMOylation of K1111 lysine residues prevents the degradation of BRD4, thereby activating the transcriptional activities of the IL-1β, TNF-α and IL-6 genes, which depend on BRD4. SUMOylated BRD4 also recruits the multifunctional methyltransferase subunit TRM112-like protein (TRMT112) to further promote the processing of proinflammatory gene transcripts to eventually increase their expression. In vivo, treatment of PTOA with a Cbx4 inhibitor in rats was comparable to treatment with BRD4 inhibitors, indicating the importance of SUMOylation in controlling BRD4 to alleviate PTOA. Overall, this study is the first to identify Cbx4 as the enzyme responsible for the SUMO modification of BRD4 and highlights the central role of the Cbx4-BRD4 axis in exacerbating PTOA from the perspective of inflammation. In research on post-traumatic osteoarthritis, scientists found a lack of knowledge on how inflammation worsens the disease. Researchers found that the protein BRD4, when altered by a process called SUMOylation, is crucial in causing inflammation in PTOA. The study used human tissue and rats to see how blocking BRD4 and a related protein, Cbx4, affects inflammation and disease development. The study involved 45 rats and examined how these proteins contribute to PTOA, using various techniques to understand their roles in inflammation. The findings showed that blocking BRD4 and Cbx4 lessened inflammation and joint damage in rats with PTOA. Specifically, treatments targeting these proteins lowered inflammation markers and improved joint health. The team concluded that the BRD4-Cbx4 interaction is a major factor in PTOA inflammation and progression, marking a significant step in understanding the disease’s molecular workings. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
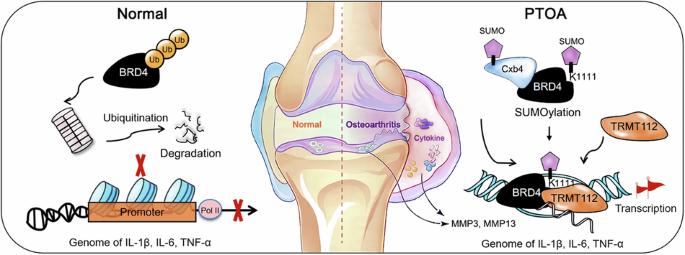
Cbx4 SUMOylates BRD4 可调节创伤后骨关节炎中炎性细胞因子的表达。
溴化结构域蛋白 4(BRD4)是一种染色质阅读器,已知它能通过控制炎性细胞因子的表达来加剧创伤后骨关节炎(PTOA)的炎症反应。然而,BRD4 翻译后这种调控作用的改变程度在很大程度上仍是未知数。在这项研究中,我们发现E3 SUMO蛋白连接酶CBX4(Cbx4)参与了BRD4的SUMO修饰,从而影响其控制滑膜成纤维细胞中促炎基因IL-1β、TNF-α和IL-6的表达。具体来说,Cbx4 介导的 K1111 赖氨酸残基 SUMO 化阻止了 BRD4 的降解,从而激活了依赖于 BRD4 的 IL-1β、TNF-α 和 IL-6 基因的转录活性。SUMOylated BRD4 还会招募多功能甲基转移酶亚基 TRM112-like 蛋白(TRMT112),进一步促进促炎基因转录本的加工,最终增加其表达。在体内,用 Cbx4 抑制剂治疗大鼠的 PTOA 与用 BRD4 抑制剂治疗的效果相当,这表明 SUMOylation 在控制 BRD4 以缓解 PTOA 方面的重要性。总之,这项研究首次确定了 Cbx4 是负责对 BRD4 进行 SUMO 修饰的酶,并从炎症的角度强调了 Cbx4-BRD4 轴在加重 PTOA 中的核心作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


