Hydroxylamines: From Synthetic Intermediates to Synthetic Targets
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
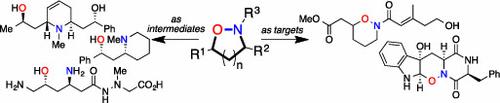
Synergy between the teaching and research activities of a University should be a source of new ideas, each informing the other. A classroom discussion gave rise to our concept of using hydroxylamines as a form of “tethered nitrogen” for alkaloid synthesis. The “tether” temporarily connects a nucleophilic nitrogen atom to the substrate, rendering an intermolecular reaction intramolecular, thus providing stereo- and regiochemical control for C–N bond formation. In the context of the synthesis of 1,3-amino alcohols, this necessitated the synthesis of isoxazolidines. This concept led to the exploration of methods for the synthesis of these heterocycles beyond the well-established 1,3-dipolar cycloadditions. The first two methods developed based upon this concept were palladium catalyzed cyclocarbonylation and silver catalyzed allene cyclization. These new methods were applied to two syntheses of sedamine and the synthesis of three Nuphar alkaloids. Intramolecular aza-Michael addition, combined with the use of cross-metathesis to generate the substrates, gave access to both isoxazolidines and tetrahydro-1,2-oxazines. This new method made possible a synthesis of monomorine with high stereochemical control. The extension to more complex alkaloids required the incorporation of additional chemistry. Combining allene cyclization with iminium ion chemistry allowed the extension to the more complex piperidine alkaloids porantheridine and sedinine. In these cases, successful stereocontrol relied on the ability to predict the conformation of the intermediates and the trajectory of the nucleophilic attack, which may be sterically or stereoelectronically controlled. Inspection of our collection of methods revealed that we had good methods for cis-3,5-disubstituted isoxazolidines and trans-3,6-disubstituted tetrahydro-1,2-oxazines. Inspired by earlier work involving iminium ions, methods were developed to provide the complementary trans-isoxazolidines and cis-oxazines, using an allylation reaction. This, combined with our interest in applications of hydroformylation, led to the synthesis of 5-hydroxysedamine. Venturing away from piperidines, this method was successfully applied to the synthesis of the β-amino acid antibiotic negamycin. Use of N,O-heterocycles as intermediates naturally sparked an interest in N,O-heterocycles as synthetic targets, as there are many natural products that contain the hydroxylamine moiety, often incorporated into an isoxazolidine or 1,2-oxazine ring. As the presence of the hydroxylamine moiety cannot be fully demonstrated using the usual spectroscopic methods, total synthesis can be employed to bridge this logical gap. A synthesis of raistrickindole A, utilizing an intramolecular Mitsunobu reaction of a hydroxamic acid to form a 1,2-oxazine, confirmed the structure of this diketopiperazine natural product. A synthesis of preisomide, using an aza-Michael addition to form the required 1,2-oxazine, also confirmed the structure of this natural product.
羟胺:从合成中间体到合成靶标
大学的教学和研究活动之间的协同作用应该是新思路的源泉,两者相互启发。通过课堂讨论,我们提出了使用羟胺作为生物碱合成的一种 "系氮 "形式的概念。系链 "将一个亲核氮原子与底物暂时连接起来,使分子间反应成为分子内反应,从而为 C-N 键的形成提供立体和区域化学控制。在合成 1,3-氨基醇的过程中,这就需要合成异噁唑烷。这一概念促使人们开始探索合成这些杂环的方法,而不仅仅局限于成熟的 1,3-二极环加成法。根据这一概念开发的前两种方法是钯催化环羰基化和银催化异烯环化。这些新方法被应用于两种镇静胺的合成和三种 Nuphar 生物碱的合成。分子内的偶氮-迈克尔加成法结合使用交叉甲基化反应生成底物,可以得到异噁唑烷和四氢-1,2-噁嗪。这一新方法使高度立体化学控制的单吗啉合成成为可能。要扩展到更复杂的生物碱,就需要加入更多的化学成分。将烯环化与亚氨基离子化学结合起来,就可以扩展到更复杂的哌啶生物碱卟吩醚啶和沉香碱。在这些情况下,成功的立体控制依赖于预测中间体构象和亲核攻击轨迹的能力,这可能是立体或立体电子控制的结果。对我们收集的方法进行检查后发现,我们已经掌握了顺式-3,5-二取代异噁唑烷和反式-3,6-二取代四氢-1,2-噁嗪的良好方法。受先前涉及亚氨基离子工作的启发,我们开发出了利用烯丙基化反应提供互补的反式异噁唑烷和顺式噁嗪的方法。再加上我们对氢甲酰化应用的兴趣,最终合成了 5-羟基苯乙胺。从哌啶类化合物出发,我们成功地将这种方法应用到了β-氨基酸抗生素奈达霉素的合成中。使用 N,O-杂环作为中间体自然引发了人们对 N,O-杂环作为合成目标的兴趣,因为有许多天然产物都含有羟胺分子,通常与异噁唑烷或 1,2- 嗪环结合在一起。由于羟胺分子的存在无法通过通常的光谱方法得到充分证明,因此可以采用全合成方法来弥补这一逻辑差距。利用羟肟酸分子内 Mitsunobu 反应生成 1,2-恶嗪,合成了雷司吲哚 A,证实了这种二酮哌嗪天然产物的结构。利用氮杂迈克尔加成法合成普利索米,形成所需的 1,2-恶嗪,也证实了这种天然产物的结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


