Discovery and Functional Characterization of a Potent, Selective, and Metabolically Stable PROTAC of the Protein Kinases DYRK1A and DYRK1B
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Small-molecule-induced protein degradation has emerged as a promising pharmacological modality for inactivating disease-relevant protein kinases. DYRK1A and DYRK1B are closely related protein kinases that are involved in pathological processes such as neurodegeneration, cancer development, and adaptive immune homeostasis. Herein, we report the development of the first DYRK1 proteolysis targeting chimeras (PROTACs) that combine a new ATP-competitive DYRK1 inhibitor with ligands for the E3 ubiquitin ligase component cereblon (CRBN) to induce ubiquitination and subsequent proteasomal degradation of DYRK1A and DYRK1B. The lead compound (DYR684) promoted fast, efficient, potent, and selective degradation of DYRK1A in cell-based assays. Interestingly, an enzymatically inactive splicing variant of DYRK1B (p65) resisted degradation. Compared to competitive kinase inhibition, targeted degradation of DYRK1 by DYR684 provided improved suppression of downstream signaling. Collectively, our results identify DYRKs as viable targets for PROTAC-mediated degradation and qualify DYR684 as a useful chemical probe for DYRK1A and DYRK1B.
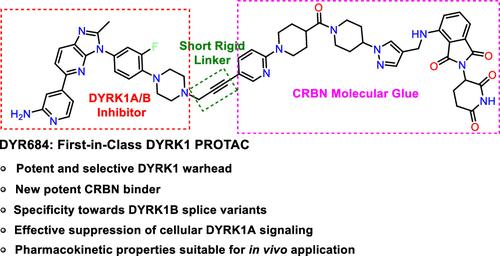
蛋白激酶 DYRK1A 和 DYRK1B 的强效、选择性和代谢稳定的 PROTAC 的发现和功能表征
小分子诱导的蛋白质降解已成为一种很有前景的药理学模式,可使与疾病相关的蛋白激酶失活。DYRK1A 和 DYRK1B 是密切相关的蛋白激酶,它们参与了神经变性、癌症发展和适应性免疫稳态等病理过程。在此,我们报告了首个 DYRK1 蛋白质溶解靶向嵌合体(PROTACs)的开发情况,该嵌合体结合了一种新的 ATP 竞争性 DYRK1 抑制剂和 E3 泛素连接酶成分 cereblon (CRBN) 的配体,以诱导 DYRK1A 和 DYRK1B 的泛素化和随后的蛋白酶体降解。在基于细胞的试验中,先导化合物(DYR684)促进了 DYRK1A 快速、高效、强效和选择性降解。有趣的是,DYRK1B 的一种无酶活性的剪接变体(p65)却能抵抗降解。与竞争性激酶抑制相比,DYR684 对 DYRK1 的靶向降解改善了对下游信号的抑制。总之,我们的研究结果确定了 DYRKs 是 PROTAC 介导的降解的可行靶标,并使 DYR684 成为 DYRK1A 和 DYRK1B 的有用化学探针。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


