Proinflammatory immune cells disrupt angiogenesis and promote germinal matrix hemorrhage in prenatal human brain
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
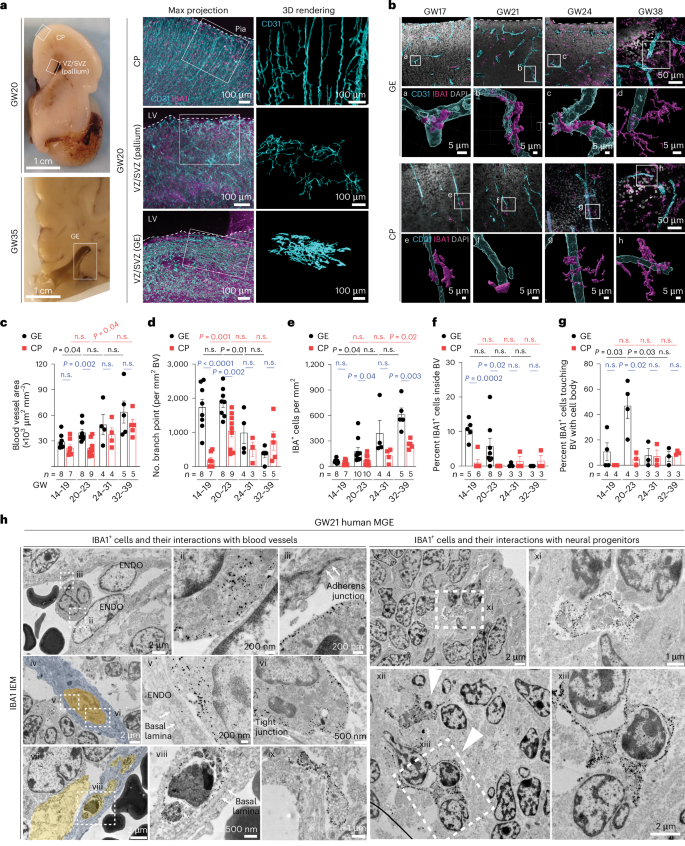
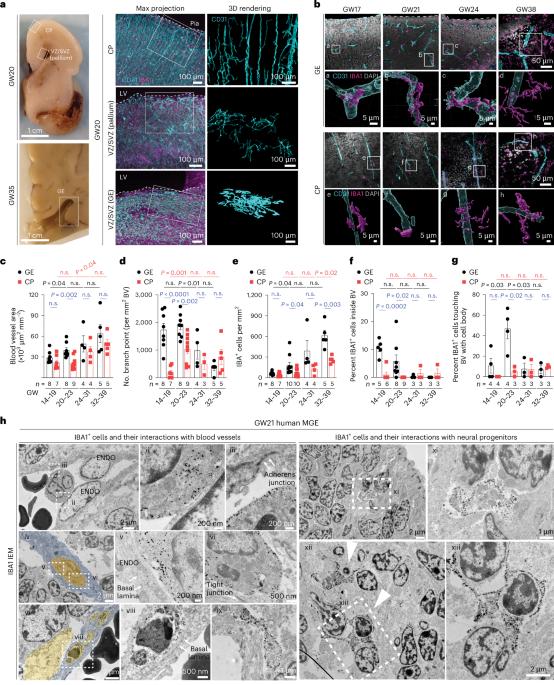
Germinal matrix hemorrhage (GMH) is a devastating neurodevelopmental condition affecting preterm infants, but why blood vessels in this brain region are vulnerable to rupture remains unknown. Here we show that microglia in prenatal mouse and human brain interact with nascent vasculature in an age-dependent manner and that ablation of these cells in mice reduces angiogenesis in the ganglionic eminences, which correspond to the human germinal matrix. Consistent with these findings, single-cell transcriptomics and flow cytometry show that distinct subsets of CD45+ cells from control preterm infants employ diverse signaling mechanisms to promote vascular network formation. In contrast, CD45+ cells from infants with GMH harbor activated neutrophils and monocytes that produce proinflammatory factors, including azurocidin 1, elastase and CXCL16, to disrupt vascular integrity and cause hemorrhage in ganglionic eminences. These results underscore the brain’s innate immune cells in region-specific angiogenesis and how aberrant activation of these immune cells promotes GMH in preterm infants. Chen et al. show that subtypes of immune cells in prenatal human brain promote angiogenesis in the germinal matrix. Conversely, in preterm infants, proinflammatory immune cells disrupt angiogenesis and promote germinal matrix hemorrhage.
前炎症性免疫细胞破坏血管生成并促进产前人脑胚芽基质出血
胚芽基质出血(GMH)是一种影响早产儿神经发育的破坏性疾病,但该脑区的血管为何容易破裂仍是未知数。在这里,我们发现产前小鼠和人类大脑中的小胶质细胞以年龄依赖性的方式与新生血管相互作用,而且小鼠中这些细胞的消融会减少神经节突起的血管生成,而神经节突起与人类的生发基质相对应。与这些发现一致,单细胞转录组学和流式细胞术显示,来自对照早产儿的不同CD45+细胞亚群采用不同的信号机制来促进血管网络的形成。与此相反,GMH 婴儿的 CD45+ 细胞中含有活化的中性粒细胞和单核细胞,它们会产生促炎因子,包括氮芥 1、弹性蛋白酶和 CXCL16,从而破坏血管完整性并导致神经节突起出血。这些结果凸显了大脑先天性免疫细胞在特定区域血管生成中的作用,以及这些免疫细胞的异常激活是如何促进早产儿GMH的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


