Optimization of Potent, Efficacious, Selective and Blood–Brain Barrier Penetrating Inhibitors Targeting EGFR Exon20 Insertion Mutations
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
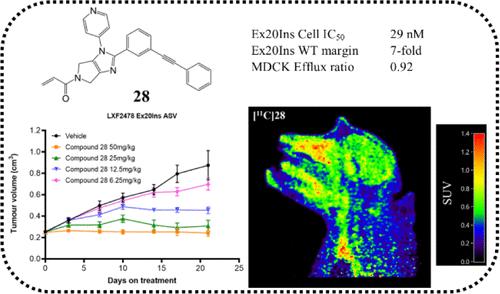
Herein, we report the optimization of a series of epidermal growth factor receptor (EGFR) Exon20 insertion (Ex20Ins) inhibitors using structure-based drug design (SBDD), leading to the discovery of compound 28, a potent and wild type selective molecule, which demonstrates efficacy in multiple EGFR Ex20Ins xenograft models and blood–brain barrier penetration in preclinical species. Building on our earlier discovery of an in vivo probe, SBDD was used to design a novel bicyclic core with a lower molecular weight to facilitate blood–brain barrier penetration. Further optimization including strategic linker replacement and diversification of the ring system interacting with the c-helix enabled photolytic and metabolic stability improvements. Together with refinement of molecular properties important for achieving high brain exposure, including molecular weight, H-bonding, and polarity, 28 was identified.
优化针对表皮生长因子受体外显子 20 插入突变的强效、有效、选择性和血脑屏障穿透性抑制剂
在本文中,我们报告了利用基于结构的药物设计(SBDD)对一系列表皮生长因子受体(EGFR)Exon20 插入(Ex20Ins)抑制剂进行优化的结果,最终发现了化合物 28,这是一种强效的野生型选择性分子,在多种 EGFR Ex20Ins 异种移植模型中显示出疗效,并在临床前物种中显示出血脑屏障穿透性。在早先发现的体内探针的基础上,我们利用 SBDD 设计了一种新型双环核心,分子量较低,有利于血脑屏障穿透。通过进一步优化,包括策略性地更换连接体以及使与 c-螺旋相互作用的环系统多样化,光解稳定性和代谢稳定性都得到了提高。此外,还完善了对实现高脑部暴露率非常重要的分子特性,包括分子量、H 键和极性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


