Mixtures of Intrinsically Disordered Neuronal Protein Tau and Anionic Liposomes Reveal Distinct Anionic Liposome-Tau Complexes Coexisting with Tau Liquid–Liquid Phase-Separated Coacervates
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
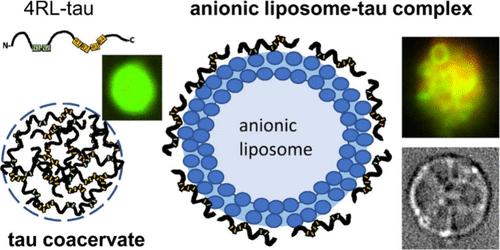
Tau, an intrinsically disordered neuronal protein and polyampholyte with an overall positive charge, is a microtubule (MT) associated protein that binds to anionic domains of MTs and suppresses their dynamic instability. Aberrant tau-MT interactions are implicated in Alzheimer’s and other neurodegenerative diseases. Here, we studied the interactions between full-length human protein tau and other negatively charged binding substrates, as revealed by differential interference contrast (DIC) and fluorescence microscopy. As a binding substrate, we chose anionic liposomes (ALs) containing either 1,2-dioleoyl-sn-glycero-3-phosphatidylserine (DOPS, −1e) or 1,2-dioleoyl-sn-glycero-3-phosphatidylglycerol (DOPG, −1e) mixed with zwitterionic 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) to mimic anionic plasma membranes of axons where tau resides. At low salt concentrations (0 to 10 mM KCl or NaCl) with minimal charge screening, reaction mixtures of tau and ALs resulted in the formation of distinct states of AL-tau complexes coexisting with liquid–liquid phase-separated tau self-coacervates arising from the polyampholytic nature of tau containing cationic and anionic domains. AL-tau complexes (i.e. tau-lipoplexes) exhibited distinct types of morphologies. This included large ∼20–30 μm tau-decorated giant vesicles with additional smaller liposomes with bound tau attached to the giant vesicles and tau-mediated finite-size assemblies of small liposomes. As the salt concentration was increased to near and above 150 mM for 1:1 electrolytes, AL-tau complexes remained stable, while tau self-coacervate droplets were found to dissolve, indicative of the breaking of (anionic/cationic) electrostatic bonds between tau chains due to increased charge screening. The findings are consistent with the hypothesis that distinct cationic domains of tau may interact with anionic lipid domains of the lumen-facing monolayer of the axon’s plasma membrane, suggesting the possibility of transient yet robust interactions near relevant ionic strengths found in neurons.
本质上紊乱的神经元蛋白 Tau 与阴离子脂质体的混合物揭示了与 Tau 液-液相分离共存的独特阴离子脂质体-Tau 复合物
Tau是一种内在紊乱的神经元蛋白质,也是一种多聚电解质,带有整体正电荷,是一种与微管(MT)相关的蛋白质,能与MT的阴离子结构域结合,抑制其动态不稳定性。tau-MT相互作用的异常与阿尔茨海默氏症和其他神经退行性疾病有关。在这里,我们通过微分干涉对比(DIC)和荧光显微镜,研究了全长人类蛋白 tau 与其他带负电荷的结合底物之间的相互作用。作为结合底物,我们选择了含有 1,2-二油酰-sn-甘油-3-磷脂酰丝氨酸(DOPS,-1e)或 1,2-二油酰-sn-甘油-3-磷脂酰甘油(DOPG、-1e)与滋养型 1,2-二油酰-sn-甘油-3-磷脂酰胆碱(DOPC)混合,以模拟 tau 所在的轴突阴离子质膜。在盐浓度较低(0 至 10 mM KCl 或 NaCl)、电荷筛选最小的条件下,tau 和 ALs 的反应混合物会形成不同状态的 AL-tau 复合物,这些复合物与液-液相分离的 tau 自凝聚物共存。AL-tau复合物(即tau-脂质)呈现出不同类型的形态。这包括由 tau 装饰的 20 ∼ 30 μm 大的巨型囊泡,巨型囊泡上附有与 tau 结合的更小的脂质体,以及由 tau 介导的小脂质体的有限尺寸集合体。随着 1:1 电解质的盐浓度增加到接近或高于 150 mM,AL-tau 复合物保持稳定,而 tau 自凝聚液滴则被发现溶解,这表明由于电荷屏蔽增加,tau 链之间的(阴离子/阳离子)静电键断裂。这些发现与tau的不同阳离子结构域可能与轴突质膜面向管腔单层的阴离子脂质结构域相互作用的假说一致,表明在神经元中的相关离子强度附近可能存在短暂而强大的相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


