Mastering the Foreign Ionic Radius in CeO2 Supports of Ni-Based Catalysts for Efficient CO2 Methanation
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
A Ni-based catalyst is a common non-noble-metal system for CO2 methanation, and metal modification for the support is an effective method to further improve its activity. Doping metals with various ionic radii already exhibited different effects on the methanation performance, but the promotion mechanism remains indistinct. Herein, we report a universal doping strategy to fabricate Ni-based catalysts by tailoring foreign ions (Al3+, Zr4+, Y3+, and Sm4+) with different radii in CeO2. Among them, Ni/Ce0.9Al0.1Ox exhibited the highest catalytic performance (72.4% of CO2 conversion at 300 °C) compared with other catalysts at all temperatures and with the lowest kinetic temperature range. Furthermore, the change in the foreign ionic radius had a variety of effects on the structure of the catalysts, in which the specific surface area and oxygen vacancy concentration were the main factors. According to mechanical measurements, the doping of foreign ions reduced the temperature of the started methanation and inhibited CO formation, resulting in enhanced catalytic activity CO2 conversion and increased CH4 selectivity. The hydroxyl groups derived from the oxygen vacancy facilitated the transformation of CO2 to CH4 via the HCOO* path. This work contributes to developing a promising approach to controlling the performance of CO2 methanation by regulating the foreign ionic radius.
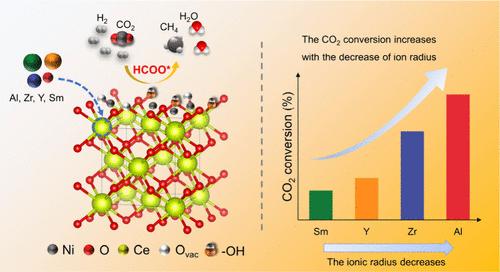
掌握镍基催化剂 CeO2 载体中的外来离子半径以实现高效 CO2 甲烷化
镍基催化剂是一种常见的二氧化碳甲烷化非贵金属体系,而对载体进行金属改性是进一步提高其活性的有效方法。掺杂不同离子半径的金属已对甲烷化性能产生了不同的影响,但其促进机制仍不明确。在此,我们报告了一种通用的掺杂策略,通过在 CeO2 中定制不同半径的外来离子(Al3+、Zr4+、Y3+ 和 Sm4+)来制造 Ni 基催化剂。其中,与其他催化剂相比,Ni/Ce0.9Al0.1Ox 在所有温度下均表现出最高的催化性能(300 ℃ 时二氧化碳转化率为 72.4%),且动力学温度范围最低。此外,外来离子半径的变化对催化剂的结构产生了多种影响,其中比表面积和氧空位浓度是主要因素。根据力学测量,外来离子的掺杂降低了开始甲烷化的温度,抑制了 CO 的形成,从而提高了催化活性 CO2 转化率,增加了 CH4 选择性。氧空位产生的羟基促进了 CO2 通过 HCOO* 途径转化为 CH4。这项工作有助于开发出一种通过调节外来离子半径来控制 CO2 甲烷化性能的可行方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


