Converting the 4-Flash Photosynthetic O2 Evolution Cycle to a 2-Flash Catalytic Cycle with a Simple Cocatalyst: Counting Electrons and Holes Directly and Transparently
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
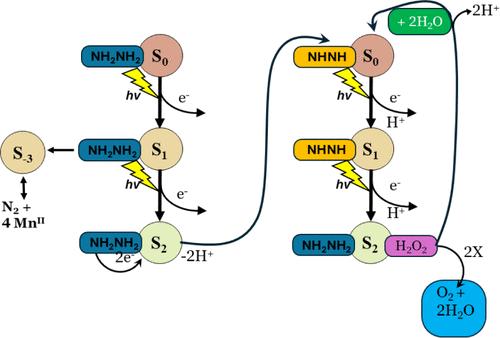
We apply a direct electron-counting method and two classes of reducing agents capable of single- vs multielectron H atom transfer reactions (nH) to probe the oxidation states of manganese in ultrapure Photosystem II (PSII) microcrystals (PSIIX) during the 4-flash catalytic cycle of O2 evolution. Flash oximetry and Fourier analysis reveal that the former class of reductants (NH2OH, H) forms two intermediates in the water oxidation center [WOC] via sequential 3H and 1H reactions, while the multi-H atom transfer class (NH2NH2, Hydrazine) operates via sequential 2H/2H reactions forming a postulated diazene intermediate (NHNH). At higher HZ concentration, a concerted 4H reaction to form N2 is favored. The diazene intermediate reacts with water via a previously unknown 2-flash O2 evolution catalytic cycle: [(S2)NHNH] + 2H2O → [NH2NH2 + (S2)H2O2] → [S0 (NHNH)] + 1/2 O2 + H2O. A lower redox state forms (S–3) by direct reduction and is 7 reducing equivalents (electrons) below the O2-evolving state (S4). This unstable state disassembles to form 4MnII + apo-WOC-PSIIX. Starting from this inactive state, an active PSIIX reforms by reconstituting the inorganic cofactors of the WOC using 7 single turnover flashes, restoring the 4-flash catalytic cycle. These results corroborate previous photoassembly studies of various PSII complexes showing that the O2-evolving S4 state is formally comprised of MnIII(MnIV)3, referred to as the low oxidation paradigm (LO). We assess incompatible interpretations of earlier spectroscopic data that assign higher oxidation states of manganese in the 4-flash catalytic cycle. We distinguish between direct and indirect experimental methods and assess their limits of applicability for assigning Mn oxidation states.
用简单的助催化剂将四闪式光合氧气进化循环转化为两闪式催化循环:直接透明地计算电子和空穴
我们采用一种直接电子计数法和两类能够进行单电子与多电子 H 原子转移反应(nH)的还原剂,来探测超纯光系统 II(PSII)微晶(PSIIX)中锰在 O2 演化的 4 闪催化循环过程中的氧化态。闪烁氧化测定法和傅立叶分析显示,前一类还原剂(NH2OH、H)通过连续的 3H 和 1H 反应在水氧化中心 [WOC] 形成两个中间产物,而多 H 原子转移类(NH2NH2、肼)则通过连续的 2H/2H 反应形成一个假定的重氮中间产物(NHNH)。在 HZ 浓度较高的情况下,形成 N2 的协同 4H 反应更受青睐。重氮中间体通过以前未知的 2 闪式 O2 进化催化循环与水发生反应:(s2)nhnh] + 2h2o → [nh2nh2 + (s2)h2o2] → [s0 (nhnh)] + 1/2 o2 + h2o。通过直接还原形成较低的氧化还原态(S-3),比 O2 生成态(S4)低 7 个还原当量(电子)。这种不稳定状态分解后形成 4MnII + apo-WOC-PSIIX。从这种非活性状态开始,活性 PSIIX 通过使用 7 次单次翻转闪烁来重组 WOC 的无机辅助因子,恢复 4 次闪烁催化循环。这些结果证实了之前对各种 PSII 复合物进行的光组装研究,研究表明 O2 演变的 S4 状态正式由 MnIII(MnIV)3 组成,被称为低氧化范式(LO)。早期的光谱数据认为锰在 4-闪烁催化循环中具有较高的氧化态,我们评估了与之不相容的解释。我们区分了直接实验方法和间接实验方法,并评估了它们在确定锰氧化态方面的适用范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


