Bidirectional pH Buffer Effect Facilitates High-Reversible Aqueous Zinc Ion Batteries
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aqueous zinc ion batteries (AZIBs) stand out from the crowd of energy storage equipment for their superior energy density, enhanced safety features, and affordability. However, the notorious side reaction in the zinc anode and the dissolution of the cathode materials led to poor cycling stability has hindered their further development. Herein, ammonium salicylate (AS) is a bidirectional electrolyte additive to promote prolonged stable cycles in AZIBs. NH4+ and C6H4OHCOO− collaboratively stabilize the pH at the interface of the electrolyte/electrode and guide the homogeneous deposition of Zn2+ at the zinc anode. The higher adsorption energy of NH4+ compared to H2O on the Zn (002) crystal plane mitigates the side reactions on the anode surface. Moreover, NH4+ is similarly adsorbed on the cathode surface, maintaining the stability of the electrode. C6H4OHCOO− and Zn2+ are co-intercalation/deintercalation during the cycling process, contributing to the higher electrochemical performance of the full cell. As a result, with the presence of AS additive, the Zn//Zn symmetric cells achieved 700 h of highly reversible cycling at 5 mA cm−2. In addition, the assembled NH4V4O10(NVO)//Zn coin and pouch batteries achieved higher capacity and higher cycle lifetime, demonstrating the practicality of the AS electrolyte additive.
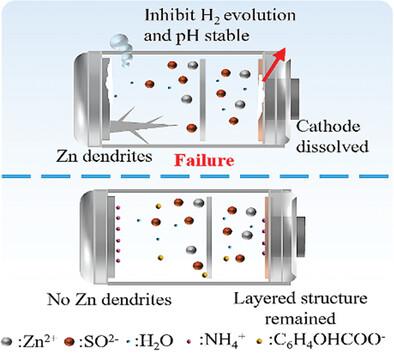
双向 pH 缓冲效应促进了锌离子水电池的高可逆性
锌离子水电池(AZIBs)以其卓越的能量密度、更强的安全性和经济性在众多储能设备中脱颖而出。然而,锌阳极中臭名昭著的副反应和阴极材料的溶解导致循环稳定性差,阻碍了其进一步发展。在此,水杨酸铵(AS)是一种双向电解质添加剂,可促进 AZIBs 的长期稳定循环。NH4+ 和 C6H4OHCOO- 可共同稳定电解液/电极界面的 pH 值,并引导 Zn2+ 在锌阳极均匀沉积。与 H2O 相比,NH4+ 在 Zn (002) 晶面上的吸附能更高,这减轻了阳极表面的副反应。此外,NH4+ 也同样吸附在阴极表面,从而保持了电极的稳定性。在循环过程中,C6H4OHCOO- 和 Zn2+ 发生共析出/脱析出,从而提高了整个电池的电化学性能。因此,在含有 AS 添加剂的情况下,Zn//Zn 对称电池在 5 mA cm-2 的条件下实现了 700 小时的高可逆循环。此外,组装后的 NH4V4O10(NVO)//Zn 纽扣电池和袋装电池实现了更高的容量和更长的循环寿命,证明了 AS 电解质添加剂的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


