Partial Solvation of Lithium Ions Enhances Conductivity in a Nanophase-Separated Polymer Electrolyte
IF 4.4
2区 化学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
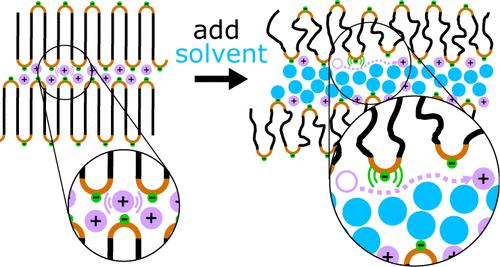
We demonstrate that a multiblock lithium-ion-conducting polymer can be swollen with ethylene carbonate solvent to increase the conductivity relative to the dry polymer material by nearly 4 orders of magnitude. This increase is due to the partial solvation of lithium ions by ethylene carbonate, which leads to Li+ diffusion along the solvent–polymer interface. This differs from the vehicular transport mechanism for lithium ions in pure solvent. We use a combination of broadband dielectric spectroscopy, X-ray scattering, and all-atom molecular dynamics simulations to probe the effect of the solvent on the polymer morphology and to elucidate the mechanism of lithium ion transport.
锂离子的部分溶解增强了纳米相分离聚合物电解质的导电性
我们证明,用碳酸乙烯酯溶剂溶胀多嵌段锂离子传导聚合物,可使其传导性相对于干聚合物材料提高近 4 个数量级。这种增加是由于碳酸乙烯酯对锂离子的部分溶解,从而导致 Li+ 沿溶剂-聚合物界面扩散。这不同于锂离子在纯溶剂中的车载传输机制。我们结合使用了宽带介电光谱、X 射线散射和全原子分子动力学模拟来探究溶剂对聚合物形态的影响,并阐明锂离子的传输机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Polymer Materials
Multiple-
CiteScore
7.20
自引率
6.00%
发文量
810
期刊介绍:
ACS Applied Polymer Materials is an interdisciplinary journal publishing original research covering all aspects of engineering, chemistry, physics, and biology relevant to applications of polymers.
The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrates fundamental knowledge in the areas of materials, engineering, physics, bioscience, polymer science and chemistry into important polymer applications. The journal is specifically interested in work that addresses relationships among structure, processing, morphology, chemistry, properties, and function as well as work that provide insights into mechanisms critical to the performance of the polymer for applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


