Engineered endothelium-mimicking antithrombotic surfaces via combination of nitric oxide-generation with fibrinolysis strategies
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
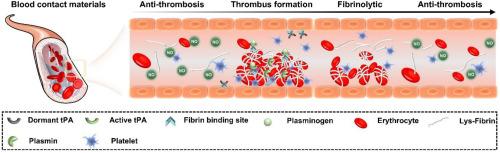
Thrombosis associated with implants can severely impact therapeutic outcomes and lead to increased morbidity and mortality. Thus, developing blood-contacting materials with superior anticoagulant properties is essential to prevent and mitigate device-related thrombosis. Herein, we propose a novel single-molecule multi-functional strategy for creating blood-compatible surfaces. The synthesized azide-modified Cu-DOTA-(Lys)3 molecule, which possesses both NO release and fibrinolysis functions, was immobilized on material surfaces via click chemistry. Due to the specificity, rapidity, and completeness of click chemistry, the firmly grafted Cu-DOTA-(Lys)3 endows the modified material with excellent antithrombotic properties of vascular endothelium and thrombolytic properties of fibrinolytic system. This surface effectively prevented thrombus formation in both in vitro and in vivo experiments, owing to the synergistic effect of anticoagulation and thrombolysis. Moreover, the modified material maintained its functional efficacy after one month of PBS immersion, demonstrating excellent stability. Overall, this single-molecule multifunctional strategy may become a promising surface engineering technique for blood-contacting materials.
一氧化氮生成与纤维蛋白溶解策略相结合的工程内皮模拟抗血栓表面
与植入物相关的血栓会严重影响治疗效果,导致发病率和死亡率上升。因此,开发具有优异抗凝特性的血液接触材料对于预防和减轻与植入物相关的血栓形成至关重要。在此,我们提出了一种新型单分子多功能策略,用于制造血液兼容表面。合成的叠氮修饰 Cu-DOTA-(Lys)3分子同时具有氮氧化物释放和纤溶功能,通过点击化学固定在材料表面。由于点击化学的特异性、快速性和完整性,牢固接枝的 Cu-DOTA-(Lys)3 使改性材料具有优异的血管内皮抗血栓性能和纤维蛋白溶解系统的溶栓性能。由于抗凝和溶栓的协同作用,这种表面在体外和体内实验中都能有效防止血栓形成。此外,经过改性的材料在浸泡 PBS 一个月后仍能保持其功能功效,显示出极佳的稳定性。总之,这种单分子多功能策略可能成为一种很有前途的血液接触材料表面工程技术。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


