Electrocatalytic cleavage of a lignin β-O-4 model compound and coupling with nitrogen-containing aromatics using Prussian blue analogue-derived nickel–cobalt spinel
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
Electrochemical conversion of lignin for the production of high-value heterocyclic aromatic compounds has great potential. We demonstrate the targeted synthesis and cation modulation of NiCo2O4 spinel nanoboxes, synthesized via cation exchange and calcination oxidation. These catalysts exhibit excellent efficacy in the electrocatalytic conversion of lignin model compounds, specifically 2-phenoxy-1-phenylethanol, into nitrogen-containing aromatics, achieving high conversion rates and selectivities. These catalysts were synthesized via a cation exchange and calcination oxidation process, using Prussian blue nanocubes as precursors. The porous architecture and polymetallic composition of the NiCo2O4 spinel demonstrated superior performance in electrocatalytic oxidative coupling, achieving a 99.2 wt% conversion rate of the 2-phenoxy-1-phenylethanol with selectivities of 37.5 wt% for quinoline derivatives and 31.5 wt% for phenol. Key innovations include the development of a sustainable one-pot synthesis method for quinoline derivatives, the elucidation of a multistage reaction pathway involving C![]() O bond cleavage, hydroxyaldol condensation, and C
O bond cleavage, hydroxyaldol condensation, and C![]() N bond formation, and a deeper mechanistic understanding derived from DFT simulations. This work establishes a new strategy for lignin valorization, offering a sustainable route to produce high-value nitrogen-containing aromatics from renewable biomass under mild conditions, without the need for additional reagents.
N bond formation, and a deeper mechanistic understanding derived from DFT simulations. This work establishes a new strategy for lignin valorization, offering a sustainable route to produce high-value nitrogen-containing aromatics from renewable biomass under mild conditions, without the need for additional reagents.
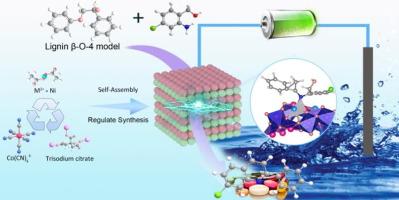
使用普鲁士蓝类似物衍生的镍钴尖晶石电催化裂解木质素 β-O-4 模型化合物并与含氮芳烃耦合
电化学转化木质素以生产高价值的杂环芳香化合物具有巨大的潜力。我们展示了通过阳离子交换和煅烧氧化合成的 NiCo2O4 尖晶石纳米盒的定向合成和阳离子调控。这些催化剂在木质素模型化合物(特别是 2-苯氧基-1-苯乙醇)电催化转化为含氮芳烃的过程中表现出卓越的功效,实现了高转化率和高选择性。这些催化剂是以普鲁士蓝纳米立方体为前驱体,通过阳离子交换和煅烧氧化工艺合成的。NiCo2O4 尖晶石的多孔结构和多金属成分在电催化氧化偶联中表现出卓越的性能,2-苯氧基-1-苯乙醇的转化率达到 99.2 wt%,对喹啉衍生物的选择性为 37.5 wt%,对苯酚的选择性为 31.5 wt%。主要创新点包括:开发了一种可持续的喹啉衍生物一锅合成方法;阐明了涉及 CO 键裂解、羟基甲醛缩合和 CN 键形成的多级反应途径;以及通过 DFT 模拟加深了对机理的理解。这项工作确立了木质素价值化的新策略,为在温和条件下从可再生生物质中生产高价值的含氮芳烃提供了一条可持续的途径,而且不需要额外的试剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


