Fabrication of carbon-supported Al2O3 nanoparticles via spontaneous cross-linking to enhance selective hydrogenation of furfural
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
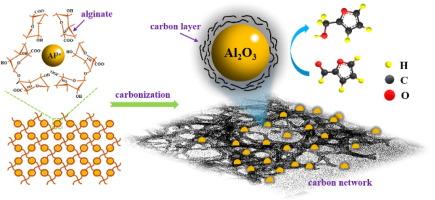
Selective hydrogenation of furfural to furfuryl alcohol is a great challenge in the hydrogenation field due to thermodynamic preference for hydrogenation of C![]() C over C
C over C![]() O. Herein, a novel Al2O3/C-u hybrid catalyst, composed of N-modified dendritic carbon networks supporting Al2O3 nanoparticles, was successfully prepared via carbonizing the freeze-dried gel from spontaneous cross-linking of alginate, Al3+ and urea. The obtained carbon-supported Al2O3 hybrid catalyst has a high ratio (31%) of Al species in pentahedral-coordinated state. The introduction of urea enhances the surface N content, the ratio of pyrrolic N, and specific surface area of catalyst, leading to improved adsorption capacity of C
O. Herein, a novel Al2O3/C-u hybrid catalyst, composed of N-modified dendritic carbon networks supporting Al2O3 nanoparticles, was successfully prepared via carbonizing the freeze-dried gel from spontaneous cross-linking of alginate, Al3+ and urea. The obtained carbon-supported Al2O3 hybrid catalyst has a high ratio (31%) of Al species in pentahedral-coordinated state. The introduction of urea enhances the surface N content, the ratio of pyrrolic N, and specific surface area of catalyst, leading to improved adsorption capacity of C![]() O and the accessibility of active sites. In the furfural hydrogenation reaction with isopropyl alcohol as hydrogen donor, Al2O3/C-u catalyst achieved a 90% conversion of furfural with 98.0% selectivity to furfuryl alcohol, outperforming that of commercial γ-Al2O3. Moreover, Al2O3/C-u demonstrates excellent catalytic stability in the recycling tests attributed to the synergistic effect of abundant weak Lewis acid sites and the anchoring effect of the carbon network on Al2O3 nanoparticles. This work provides an innovative and facile strategy for fabrication of carbon-supported Al2O3 hybrid catalysts with rich AlV species, serving as a high selective hydrogenation catalyst through MPV reaction route.
O and the accessibility of active sites. In the furfural hydrogenation reaction with isopropyl alcohol as hydrogen donor, Al2O3/C-u catalyst achieved a 90% conversion of furfural with 98.0% selectivity to furfuryl alcohol, outperforming that of commercial γ-Al2O3. Moreover, Al2O3/C-u demonstrates excellent catalytic stability in the recycling tests attributed to the synergistic effect of abundant weak Lewis acid sites and the anchoring effect of the carbon network on Al2O3 nanoparticles. This work provides an innovative and facile strategy for fabrication of carbon-supported Al2O3 hybrid catalysts with rich AlV species, serving as a high selective hydrogenation catalyst through MPV reaction route.
通过自发交联制备碳支撑 Al2O3 纳米粒子,以提高糠醛的选择性氢化能力
由于热力学上 CC 比 CO 优先加氢,因此将糠醛选择性加氢为糠醇是加氢领域的一大挑战。本文通过对海藻酸盐、Al3+ 和尿素自发交联的冻干凝胶进行碳化,成功制备了一种新型 Al2O3/C-u 混合催化剂,该催化剂由支撑 Al2O3 纳米颗粒的 N 改性树枝状碳网络组成。所获得的碳支撑 Al2O3 混合催化剂中,五面体配位状态的 Al 种类比例较高(31%)。尿素的引入提高了催化剂的表面 N 含量、吡咯烷 N 比例和比表面积,从而提高了 CO 的吸附能力和活性位点的可及性。在以异丙醇为供氢体的糠醛加氢反应中,Al2O3/C-u 催化剂的糠醛转化率达到 90%,对糠醇的选择性达到 98.0%,优于商用 γ-Al2O3 催化剂。此外,Al2O3/C-u 在循环测试中表现出优异的催化稳定性,这归功于丰富的弱路易斯酸位点和碳网络对 Al2O3 纳米颗粒的锚定作用的协同效应。这项工作为制备富含 AlV 物种的碳支撑 Al2O3 混合催化剂提供了一种创新而简便的策略,可通过 MPV 反应路线用作高选择性加氢催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


