Rapid formation of carbon dioxide hydrate governed by the natural nano-clay for effective carbon dioxide capture
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
Gas hydrate can separate and capture enormous carbon dioxide (CO2) only using water cages. Capturing CO2 in hydrate form is viewed as a green and effective technology to reduce the global CO2 emission. However, the sluggish kinetics and high cost of kinetics promotors of CO2 hydrate formation limited the application of hydrate-based CO2 capture technology. Here, we used the nano-clay prepared by cheap natural clay to promote the formation kinetics of CO2 hydrate. At the low concentration range, the formation period of CO2 hydrate in the two-dimensional nano-clay dispersion was significantly shorter than that of the one-dimensional nano-clay dispersion. The induction time (5 min) obtained in vermiculite nanoflakes dispersion was shorter than most solid promotors of CO2 hydrate formation kinetics in reported works. Besides, we found that the water molecules near the negatively charged two-dimensional nano-clay surface were more likely to form the cage-like structure than those around the one-dimensional nano-clay surface. The similar hydrogen-bonded structure of the cage-like water with CO2 hydrate made them favorable for the CO2 hydrate formation. These results shed light on the design of the effective kinetics promotors of CO2 hydrate and contribute to develop the hydrate-based CO2 capture technologies.
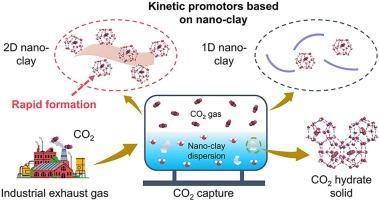
利用天然纳米粘土快速形成二氧化碳水合物,有效捕获二氧化碳
气体水合物只能利用水笼分离和捕获巨大的二氧化碳(CO2)。以水合物形式捕获二氧化碳被视为减少全球二氧化碳排放的一项绿色有效技术。然而,二氧化碳水合物形成的动力学缓慢且动力学促进剂成本高昂,限制了基于水合物的二氧化碳捕获技术的应用。在此,我们利用廉价天然粘土制备的纳米粘土来促进二氧化碳水合物的形成动力学。在低浓度范围内,二维纳米粘土分散体中 CO2 水合物的形成周期明显短于一维纳米粘土分散体。在蛭石纳米片分散液中获得的诱导时间(5 分钟)短于大多数已报道的二氧化碳水合物形成动力学固体促进剂。此外,我们还发现带负电荷的二维纳米粘土表面附近的水分子比一维纳米粘土表面附近的水分子更容易形成笼状结构。笼状水与二氧化碳水合物的氢键结构相似,因此有利于二氧化碳水合物的形成。这些结果为设计有效的二氧化碳水合物动力学促进剂提供了启示,有助于开发基于水合物的二氧化碳捕获技术。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


