Synthesis of silacycles via metal hydrogen atom transfer and radical-polar crossover mechanism
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this study, we have developed an efficient method for silacycle synthesis using metal hydride hydrogen atom transfer (MHAT) and radical-polar crossover (RPC) mechanism. Allylic silanols were synthesized via one-step condensation and then cyclized under mild conditions using N-fluoropyridinium tetrafluoroborate (Me3NFPY·BF4) as the oxidant under cobalt catalysis. This approach yielded five-, six-, and seven-membered silacycles with high selectivity, with the six-membered rings being the favored products. Density functional theory (DFT) calculations provided valuable insights into the reaction mechanisms and highlighted the role of ring strain in determining product selectivity. This study demonstrated the effectiveness of combining MHAT/RPC methodologies with silicon tethering, thus offering a robust platform for expanding the use of silicon-based strategies in synthetic chemistry.
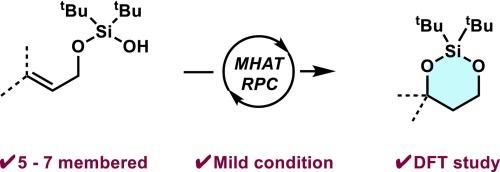
通过金属氢原子转移和自由基-极性交叉机制合成硅圈
在这项研究中,我们开发了一种利用金属氢化物氢原子转移(MHAT)和自由基-极性交叉(RPC)机制合成硅烷醇的高效方法。通过一步缩合合成了烯丙基硅烷醇,然后在钴催化下使用 N-氟吡啶鎓四氟硼酸盐(Me3NFPY-BF4)作为氧化剂在温和条件下进行环化。这种方法以高选择性生成了五元、六元和七元硅环,其中六元环是最受欢迎的产物。密度泛函理论(DFT)计算为了解反应机理提供了宝贵的见解,并突出了环应变在决定产物选择性中的作用。这项研究证明了将 MHAT/RPC 方法与硅系链相结合的有效性,从而为扩大硅基策略在合成化学中的应用提供了一个强大的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


