Adsorbate-induced adatom formation on Au-Cu bimetallic alloys and its possible consequences for CO2 electroreduction
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The adsorbate-induced formation of sub-nanometer clusters on transition-metal single crystals observed in previous high-pressure microscopic studies hinted at the in-situ formation of unique active sites even on large nanoparticle catalysts. We propose that the adatom formation energy can be used as an energetic descriptor for the initial step toward the adsorbate-induced metal-cluster formation process. This descriptor can be efficiently computed using density functional theory (DFT) calculations and applied for screening and identification of metal catalysts where this phenomenon may play an important role in generating active sites in-situ. As a proof of concept, here, we construct an adatom formation energy database for three AuxCuy alloys (x:y = 3:1, 1:1, or 1:3) and eighteen adsorbates (H, C, N, O, F, S, Cl, Br, I, CHx, NHx (x = 1 – 3), CO, NO, and OH) commonly involved in catalytic reactions. The energetics of adatom formation were examined in all cases where the (111) terrace, (211) step-edge, and (874) kink were the sources of the adatom. We demonstrate that the presence of an adsorbate could alter not only the energetics for adatom formation but also the elemental nature of the preferred adatom being formed. Using our database, we identified promising systems which favor adsorbate-induced adatom formation under near-ambient conditions. Specifically, CO-induced adatom formation on all three Au-Cu alloy surfaces could occur under CO2 electroreduction (CO2RR) conditions. This phenomenon offers a qualitative explanation for the experimentally observed CO2RR activity on Au-Cu alloy catalysts. Our methodology offers an easily expandable and efficient approach for large-scale catalyst screening with regards to adatom/cluster formation under reaction conditions and provides insight into the possible nature of active sites on alloy catalysts from a novel perspective.
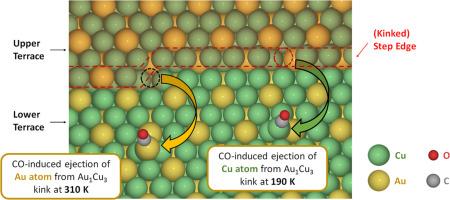
金铜双金属合金上吸附剂诱导的金刚石形成及其对二氧化碳电还原的可能影响
在以前的高压显微研究中观察到的过渡金属单晶上由吸附剂诱导形成的亚纳米簇,暗示了即使在大型纳米粒子催化剂上也能在原位形成独特的活性位点。我们提出,在吸附剂诱导的金属簇形成过程的初始步骤中,可将原子形成能用作能量描述符。这种描述符可以通过密度泛函理论(DFT)计算有效地计算出来,并应用于金属催化剂的筛选和鉴定,因为这种现象可能在原位生成活性位点的过程中发挥重要作用。作为概念验证,我们在此为三种 AuxCuy 合金(x:y = 3:1、1:1 或 1:3)和催化反应中常见的 18 种吸附剂(H、C、N、O、F、S、Cl、Br、I、CHx、NHx(x = 1 - 3)、CO、NO 和 OH)构建了一个吸附体形成能数据库。在所有以 (111) 梯面、(211) 阶边和 (874) 疙瘩为腺体来源的情况下,我们都研究了腺体形成的能量学。我们证明,吸附剂的存在不仅会改变形成金刚原子的能量,还会改变所形成的优选金刚原子的元素性质。利用我们的数据库,我们确定了在近环境条件下有利于吸附剂诱导的腺体形成的有前途的系统。具体来说,在二氧化碳电还原(CO2RR)条件下,所有三种金-铜合金表面都会出现二氧化碳诱导的腺体形成。这一现象为实验观察到的 Au-Cu 合金催化剂的 CO2RR 活性提供了定性解释。我们的方法为大规模催化剂筛选提供了一种易于扩展且高效的方法,可用于研究反应条件下的金刚石/簇的形成,并从一个新的角度深入了解合金催化剂上活性位点的可能性质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


