Control of encapsulation efficiency and morphology of poly(lactide-co-glycolide) microparticles with a diafiltration-driven solvent extraction process
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-09-24
DOI:10.1016/j.ejpb.2024.114515
引用次数: 0
Abstract
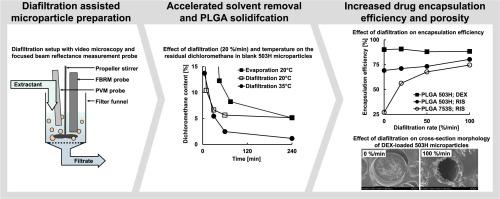
The removal of organic solvents during the preparation of biodegradable poly(D,L-lactide-co-glycolide) (PLGA) microparticles by an O/W- solvent extraction/evaporation process was investigated and controlled by diafiltration. Emulsification and steady replacement of the aqueous phase were performed in parallel in a single-vessel setup. During the process, the solidification of the dispersed phase (drug:PLGA:solvent droplets) into microparticles was monitored with video-microscopy and focused beam reflectance measurement (FBRM) and the residual solvent content was analyzed with headspace gas chromatography (organic solvent) and coulometric Karl-Fischer titration (water). Microparticles containing dexamethasone or risperidone were characterized with regard to particle size, morphology, encapsulation efficiency and in-vitro release. Diafiltration-accelerated solvent extraction shortened the process time by accelerating solidification of dispersed phase but reduced the residual dichloromethane content only in combination with increased temperature. Increasing the diafiltration rate increased particle size, porosity, and the encapsulation efficiency of risperidone. The latter effect was particularly evident with increasing lipophilicity of PLGA. A slower and more uniform solidification of end-capped and increased lactide content PLGA grade was identified as the reason for an increased drug leaching. Accelerated solvent extraction by diafiltration did not affect the in-vitro release of risperidone from different PLGA grades. The initial burst release of dexamethasone was increased by diafiltration when encapsulated in concentrations above the percolation threshold. Both porosity and burst release could be reduced by increasing the process temperature during diafiltration. Residual water content was established as an indicator for porosity and correlated with the burst release of dexamethasone.
用重过滤驱动的溶剂萃取工艺控制聚(乳酸-共聚-乙二醇)微颗粒的封装效率和形态
研究了通过 O/W- 溶剂萃取/蒸发工艺制备可生物降解的聚(D,L-乳酸-共聚乙二醇)(PLGA)微粒过程中有机溶剂的去除情况,并通过重过滤进行了控制。乳化和水相的稳定置换在单容器装置中同时进行。在此过程中,使用视频显微镜和聚焦光束反射测量法(FBRM)监测分散相(药物:PLGA:溶剂液滴)凝固成微颗粒的过程,并使用顶空气相色谱法(有机溶剂)和库仑法卡尔-费歇尔滴定法(水)分析残留溶剂含量。含有地塞米松或利培酮的微颗粒在粒度、形态、包封效率和体外释放方面均有表征。重过滤-加速溶剂萃取法通过加速分散相的凝固缩短了工艺时间,但只有在温度升高的情况下才能减少二氯甲烷的残留量。提高重滤速率可增加利培酮的粒度、孔隙率和封装效率。随着聚乳酸甘油酯亲脂性的增加,后一种效果尤为明显。药物浸出增加的原因是,端帽聚乳酸含量增加的聚乳酸凝固速度更慢、更均匀。通过重滤加速溶剂萃取不会影响不同等级 PLGA 中利培酮的体外释放。当地塞米松的封装浓度高于渗滤阈值时,重滤会增加地塞米松的初始猝灭释放。在重滤过程中提高工艺温度可降低孔隙率和猝灭释放率。残余水含量被确定为孔隙率的指标,并与地塞米松的猝发释放相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


