Rich active sites ZIF-8 base on imidazole-based deep eutectic solvents for rapid adsorption of acid fuchsin and competitive adsorption
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
MimDES-ZIF-8 with rich active sites as positive charges and metal coordination sites were prepared based on imidazole based deep eutectic solvent (MimDES) using interface permeation method. 2-Methylimidazole (2-Mim) simultaneously acted as a hydrogen bond acceptor (HBA) in MimDES (hydrogen donner was 1,6-hexanediol) and an organic ligand in MimDES-ZIF-8, affecting the coordination of ZIF-8 during permeation. Four different zinc salt solutions (ZnCl2, Zn(NO3)2·6H2O, Zn(OAc)2·2H2O, ZnSO4·7H2O) were permeated into MimDES to obtain MimDES-ZIF-8 with different morphologies. All MimDES-ZIF-8 had higher Zn/N ration (>0.25), specific surface area and surface positive charges. The dye adsorption results indicated that MimDES-ZIF-8 showed high adsorption efficiencies for acid fuchsin (all exceeded 97.75 % under pH 7.5) and a fast adsorption rate (exceeding 93 % in 5 min and reaching equilibrium in 60 min). Among MimDES-ZIF-8, the adsorption capacity of ZIF-8-C was 4845.3 mg/g. After five cycles of adsorption, the AF removal efficiency of ZIF-8-C remained over 93.23 %. Moreover, ZIF-8-C also had good adsorption capacity for anionic dyes CR, cationic dyes MB and BB. The adsorption process aligned well with both the Langmuir model and the pseudo-second-order kinetic model. Based on FTIR, XPS, and electrostatic potential calculation, the adsorption mechanism was elucidated.
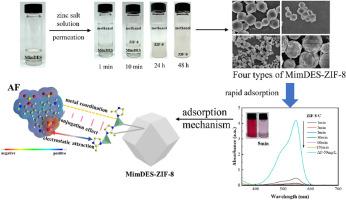
咪唑类深共晶溶剂上的富活性位点 ZIF-8 碱可快速吸附酸性品红和竞争性吸附
利用界面渗透法,在咪唑基深共晶溶剂(MimDES)的基础上制备了具有丰富正电荷活性位点和金属配位位点的 MimDES-ZIF-8。2-Methylimidazole (2-Mim) 在 MimDES 中同时充当氢键接受体(HBA)(氢捐献者为 1,6-己二醇)和 MimDES-ZIF-8 中的有机配体,在渗透过程中影响 ZIF-8 的配位。将四种不同的锌盐溶液(ZnCl2、Zn(NO3)2-6H2O、Zn(OAc)2-2H2O、ZnSO4-7H2O)渗透到 MimDES 中,得到不同形态的 MimDES-ZIF-8。所有 MimDES-ZIF-8 都具有较高的 Zn/N 比率(0.25)、比表面积和表面正电荷。染料吸附结果表明,MimDES-ZIF-8 对酸性品红的吸附效率高(在 pH 值为 7.5 的条件下均超过 97.75%),吸附速度快(5 分钟内超过 93%,60 分钟内达到平衡)。在 MimDES-ZIF-8 中,ZIF-8-C 的吸附容量为 4845.3 mg/g。经过五个吸附周期后,ZIF-8-C 对 AF 的去除率仍超过 93.23%。此外,ZIF-8-C 对阴离子染料 CR、阳离子染料 MB 和 BB 也有很好的吸附能力。吸附过程完全符合 Langmuir 模型和假二阶动力学模型。根据傅立叶变换红外光谱、XPS 和静电位计算,阐明了吸附机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


