A machine learning-based electronic nose for detecting neonatal sepsis: Analysis of volatile organic compound biomarkers in fecal samples
IF 3.2
3区 医学
Q2 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
Abstract
Background
Neonatal sepsis is a global health threat, contributing to high morbidity and mortality rates among newborns. Recognizing the profound impact of neonatal sepsis on long-term health outcomes emphasizes the critical need for timely detection to mitigate its consequences and ensure optimal health for the affected newborns. Currently, various diagnostic approaches have been implemented, but they are limited by their invasiveness, high costs, centralized testing, frequent delays, inaccuracies in results, and the need for sophisticated laboratory equipment.
Methods
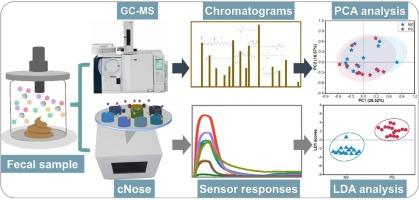
We introduced a novel, non-invasive, cost-efficient, and easy-to-use technology that can provide rapid results at a point-of-care. The technology utilized a lab-built metal oxide semiconductor-based electronic nose (cNose) combined with volatile organic compound (VOC) biomarkers identified through gas chromatography-mass spectrometry (GC–MS) analysis. The system was evaluated using fecal profiling tests involving a total of 32 samples, including 17 positive and 15 negative sepsis, confirmed by blood culture. To assess the performance in discriminating patients from healthy controls, four machine learning algorithms were implemented.
Results
Based on the cross-validation results, the MLPNN model provided the best results in distinguishing between neonates with positive and negative sepsis, achieving high-performance results of 90.63 % accuracy, 88.24 % sensitivity, and 93.33 % specificity at a 95 % confidence interval. Specific VOCs associated with neonatal sepsis, such as alcohols, acids, and esters, were successfully identified through GC–MS analysis, further validating the diagnostic capability of the cNose device.
Conclusion
The overall observations show the feasibility of using cNose system as a promising tool for real-time and bedside sepsis detection, potentially improving patient outcomes.
用于检测新生儿败血症的基于机器学习的电子鼻分析粪便样本中的挥发性有机化合物生物标记物
背景新生儿败血症是一种全球性的健康威胁,导致新生儿发病率和死亡率居高不下。认识到新生儿败血症对长期健康结果的深远影响,强调了及时发现以减轻其后果并确保受影响的新生儿获得最佳健康的迫切需要。目前,已有多种诊断方法被采用,但这些方法因其侵入性、高成本、集中检测、频繁延迟、结果不准确以及需要复杂的实验室设备而受到限制。该技术利用实验室制造的基于金属氧化物半导体的电子鼻(cNose),结合通过气相色谱-质谱(GC-MS)分析确定的挥发性有机化合物(VOC)生物标记物。该系统通过粪便分析测试进行了评估,共涉及 32 份样本,包括经血液培养确认的 17 份阳性和 15 份阴性败血症样本。结果根据交叉验证结果,MLPNN 模型在区分败血症阳性和阴性新生儿方面效果最佳,在 95% 的置信区间内达到了 90.63% 的准确率、88.24% 的灵敏度和 93.33% 的特异性。通过气相色谱-质谱分析,成功鉴定出了与新生儿败血症相关的特定挥发性有机化合物,如醇、酸和酯,进一步验证了 cNose 设备的诊断能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Clinica Chimica Acta
医学-医学实验技术
CiteScore
10.10
自引率
2.00%
发文量
1268
审稿时长
23 days
期刊介绍:
The Official Journal of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
Clinica Chimica Acta is a high-quality journal which publishes original Research Communications in the field of clinical chemistry and laboratory medicine, defined as the diagnostic application of chemistry, biochemistry, immunochemistry, biochemical aspects of hematology, toxicology, and molecular biology to the study of human disease in body fluids and cells.
The objective of the journal is to publish novel information leading to a better understanding of biological mechanisms of human diseases, their prevention, diagnosis, and patient management. Reports of an applied clinical character are also welcome. Papers concerned with normal metabolic processes or with constituents of normal cells or body fluids, such as reports of experimental or clinical studies in animals, are only considered when they are clearly and directly relevant to human disease. Evaluation of commercial products have a low priority for publication, unless they are novel or represent a technological breakthrough. Studies dealing with effects of drugs and natural products and studies dealing with the redox status in various diseases are not within the journal''s scope. Development and evaluation of novel analytical methodologies where applicable to diagnostic clinical chemistry and laboratory medicine, including point-of-care testing, and topics on laboratory management and informatics will also be considered. Studies focused on emerging diagnostic technologies and (big) data analysis procedures including digitalization, mobile Health, and artificial Intelligence applied to Laboratory Medicine are also of interest.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


