An Approach for Highly Enantioselective Synthesis of meta-Disubstituted [n]Paracyclophanes
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Atroposelective synthesis of meta-disubstituted [n]paracyclophanes is a difficult task in organic chemistry. We describe a facile approach for the synthesis of meta-disubstituted [n]paracyclophanes using Pd-catalyzed enantioselective C–H olefination and sequential reductive cleavage. A wide range of [n]paracyclophanes was obtained with excellent enantioselectivity. Thermodynamic analysis revealed that the rotational barrier of meta-disubstituted [n]paracyclophanes was lower than that of para-disubstituted [n]paracyclophanes. The synthesized planar-chiral [14]paracyclophane showed a bright fluorescence emission and impressive circularly polarized luminescence activity.
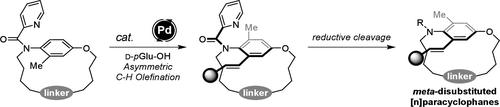
元-二取代[n]对位环烷的高对映选择性合成方法
无选择性合成元-二取代[n]对偶环烷是有机化学中的一项艰巨任务。我们介绍了一种利用 Pd 催化的对映体选择性 C-H 烯化和顺序还原裂解法合成元-二取代的 [n]paracyclophanes 的简便方法。结果获得了范围广泛的[n]对二环烷,并具有极佳的对映选择性。热力学分析表明,元二取代[n]对二环烷的旋转障碍低于对位二取代[n]对二环烷。合成的平面手性[14]对位环烷具有明亮的荧光发射和令人印象深刻的圆极化发光活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


