Mechanistic Study on Oxygen Reduction Reaction in High-Concentrated Electrolytes for Aprotic Lithium–Oxygen Batteries
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Highly concentrated electrolytes (HCEs) have energized the development of high-energy-density lithium metal batteries by facilitating the formation of robust inorganic-derived solid electrolyte interfaces on the lithium anode. However, the oxygen reduction reaction (ORR) occurring on the cathode side remains ambiguous in HCE-based lithium–oxygen (Li–O2) batteries. Herein, we investigate the ORR mechanism in a highly concentrated LiTFSI-CH3CN electrolyte using ultra-microelectrode voltammetry coupled with in situ spectroscopies. It is found that, compared to the dilute electrolyte, the HCE prolongs the lifespan of superoxide intermediates and decelerates their migration rate to the bulk solution, resulting in a change in growth mode for the discharge product of Li2O2 from traditional two-dimensional film growth to surface three-dimensional expansion growth. This alteration reduces the cathode passivation and thus delivers the enhanced discharge capacity. Additionally, the HCE also increases the reaction energy barrier between superoxide and solvent molecules, thereby minimizing parasitic reactions and improving the cycle performance of Li–O2 batteries. Our study reveals the intricate interplay between electrolytes and oxygen intermediates and provides important insights into electrolyte chemistries for better Li–O2 batteries.
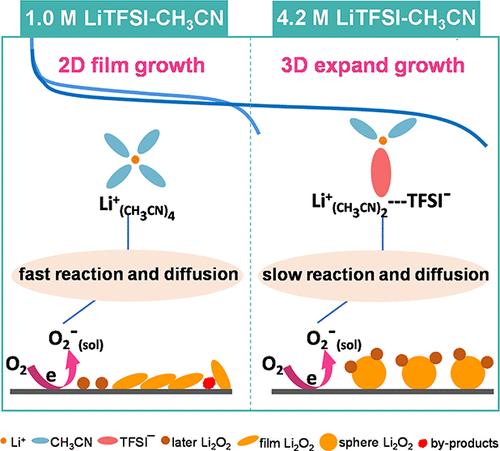
锂氧电池高浓度电解质中氧还原反应的机理研究
高浓度电解质(HCE)有利于在锂阳极上形成坚固的无机固体电解质界面,从而推动了高能量密度锂金属电池的发展。然而,在基于 HCE 的锂-氧(Li-O2)电池中,阴极侧发生的氧还原反应(ORR)仍不明确。在此,我们利用超微电极伏安法和原位光谱法研究了高浓度 LiTFSI-CH3CN 电解液中的 ORR 机理。研究发现,与稀电解质相比,高浓度锂离子电解质延长了超氧化物中间产物的寿命,并降低了它们向体液迁移的速度,从而导致锂氧化物放电产物的生长模式从传统的二维薄膜生长转变为表面三维膨胀生长。这种改变减少了阴极钝化,从而提高了放电容量。此外,HCE 还增加了超氧化物与溶剂分子之间的反应能垒,从而最大限度地减少了寄生反应,提高了锂-氧化物电池的循环性能。我们的研究揭示了电解质与氧中间产物之间错综复杂的相互作用,并为更好地开发锂-O2 电池的电解质化学提供了重要见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


