Photo-Induced Pyridylic C(sp3)–H Alkylation with Unactivated Alkenes Enabled by Hydrogen Atom Transfer/Lewis Acid Cocatalysis
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Radical hydroalkylation of alkenes represents an ideal approach for C(sp3)–C(sp3) bond formation. However, radical precursors have been substantially limited due to the sluggish nature of less electrophilic carbon-centered radicals to participate in addition step toward unactivated alkenes. Herein, we demonstrate that this inherent limitation can be overcome by the use of a Lewis acid catalyst. This cocatalytic system enables the hitherto elusive radical C(sp3)–C(sp3) bond formation between pyridylacetates and unactivated alkenes to generate medically relevant valuable products. Computational studies support that the formation of a Lewis pair with the substrates is crucial to lower the energy of the transition state for the rate-determining radical addition step.
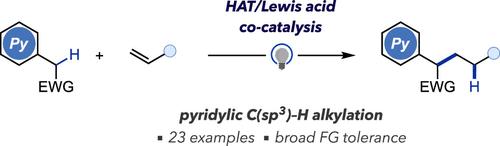
通过氢原子转移/刘易斯酸催化与未活化烯烃发生光诱导吡啶C(sp3)-H烷基化反应
烯烃的自由基氢烷基化是形成 C(sp3)-C(sp3)键的理想方法。然而,由于亲电性较弱的碳中心自由基在参与未活化烯烃的加成步骤时表现迟缓,自由基前体的作用受到了很大限制。在此,我们证明使用路易斯酸催化剂可以克服这一固有的限制。这种协同催化系统能使吡啶乙酸酯和未活化的烯烃之间形成迄今难以捉摸的 C(sp3)-C(sp3)自由基键,从而生成医学上有价值的产品。计算研究证明,与底物形成路易斯对对于降低决定速率的自由基加成步骤的过渡态能量至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


