Random epigenetic inactivation of the X-chromosomal HaMSter gene causes sex ratio distortion in persimmon
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
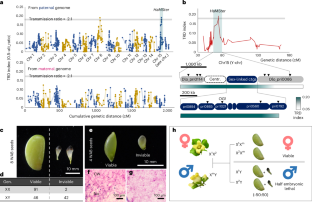
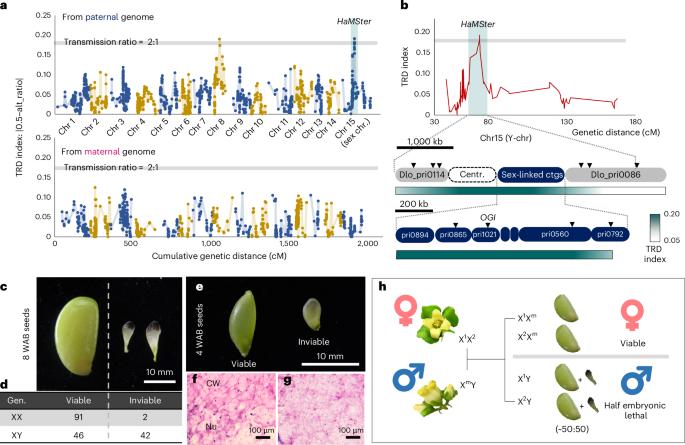
In contrast to the recent progress in the genome sequencing of plant sex chromosomes, the functional contribution of the genes in sex chromosomes remains little known1. They were classically thought to be related to sexual dimorphism, which is beneficial to male or female functions, including segregation ratios. Here we focused on the functional evolution of the sex ratio distortion-related locus Half Male Sterile/Inviable (HaMSter), which is located in the short sex-linked region in diploid persimmon (Diospyros lotus). The expression of HaMSter, encoding a plant1589-like undefined protein, is necessary for production of viable seeds. Notably, only X-allelic HaMSter is substantially expressed and half of the maternal X alleles of HaMSter is randomly inactivated, which results in sex ratio distortion in seeds. Genome-wide DNA methylome analyses revealed endosperm-specific DNA hypermethylation, especially in the X-linked region. The maintenance/release of this hypermethylation is linked to inactivation/activation of HaMSter expression, respectively, which determines the sex ratio distortion pattern. The sex ratio is often not even in plants, and its molecular mechanisms have been little known. The study found that an X chromosome-encoded gene, named HaMSter in persimmon, influences sex ratio distortion via seed viability through a regulatory mechanism involving random DNA methylation.
X 染色体 HaMSter 基因的随机表观遗传失活导致柿树性别比失真
与植物性染色体基因组测序的最新进展相比,人们对性染色体基因的功能贡献仍然知之甚少1。人们通常认为它们与性二态性有关,而性二态性有利于雄性或雌性的功能,包括分离比。在此,我们重点研究了性比畸变相关位点半雄不育/不育(HaMSter)的功能进化,该位点位于二倍体柿子(Diospyros lotus)的短性连锁区。HaMSter编码一种类似植物1589的未定义蛋白质,它的表达是产生可存活种子的必要条件。值得注意的是,只有X等位基因HaMSter大量表达,而HaMSter的母本X等位基因有一半随机失活,从而导致种子的性别比例失调。全基因组 DNA 甲基化分析显示,胚乳特异性 DNA 高甲基化,尤其是在 X 连锁区域。这种高甲基化的维持/释放分别与 HaMSter 表达的失活/活化有关,从而决定了性比畸变模式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


