Amino Acid-Based Poly(ester urea) Biodegradable Membrane for Guided Bone Regeneration
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
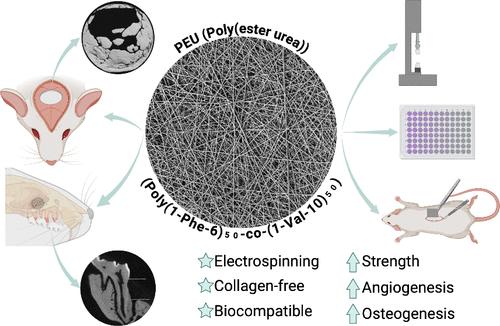
Barrier membranes (BM) for guided bone regeneration (GBR) aim to support the osteogenic healing process of a defined bony defect by excluding epithelial (gingival) ingrowth and enabling osteoprogenitor and stem cells to proliferate and differentiate into bone tissue. Currently, the most widely used membranes for these approaches are collagen-derived, and there is a discrepancy in defining the optimal collagen membrane in terms of biocompatibility, strength, and degradation rates. Motivated by these clinical observations, we designed a collagen-free membrane based on l-valine-co-l-phenylalanine-poly(ester urea) (PEU) copolymer via electrospinning. Degradation and mechanical properties of these membranes were performed on as-spun and water-aged samples. Alveolar-bone-derived stem cells (AvBMSCs) were seeded on the PEU BM to assess their cell compatibility and osteogenic characteristics, including cell viability, attachment/spreading, proliferation, and mineralized tissue-associated gene expression. In vivo, PEU BMs were subcutaneously implanted in rats to evaluate their potential to cause inflammatory responses and facilitate angiogenesis. Finally, critical-size calvarial defects and a periodontal model were used to assess the regenerative capacity of the electrospun PEU BM compared to clinically available Cytoflex synthetic membranes. PEU BM demonstrated equal biocompatibility to Cytoflex with superior mechanical performance in strength and elasticity. Additionally, after 14 days, PEU BM exhibited a higher expression of BGLAP/osteocalcin and superior in vivo performance–less inflammation and increased CD31 and VWF expression over time. When placed in critical-sized defects in the calvaria of rats, the PEU BM led to robust bone formation with high expression of osteogenesis and angiogenesis markers. Moreover, our membrane enhanced alveolar bone and cementum regeneration in an established periodontal model after 8 weeks. We demonstrate that the PEU BM exhibits favorable clinical properties, including mechanical stability, cytocompatibility, and facilitated bone formation in vitro and in vivo. This highlights its suitability for GBR in periodontal and craniofacial bone defects.
用于引导骨再生的氨基酸基聚(酯脲)生物可降解膜
用于引导骨再生(GBR)的屏障膜(BM)旨在通过阻止上皮(牙龈)生长,使骨细胞和干细胞增殖并分化为骨组织,从而支持确定骨缺损的成骨愈合过程。目前,这些方法最广泛使用的膜是胶原蛋白膜,而在生物相容性、强度和降解率方面,最佳胶原蛋白膜的定义存在差异。受这些临床观察结果的启发,我们通过电纺丝设计了一种基于 l-缬氨酸-共-l-苯丙氨酸-聚(酯脲)(PEU)共聚物的无胶原蛋白膜。这些膜的降解和机械性能在原样和水老化样品上进行了测试。将肺泡骨源性干细胞(AvBMSCs)播种在 PEU BM 上,以评估其细胞兼容性和成骨特性,包括细胞活力、附着/扩散、增殖和矿化组织相关基因的表达。在体内,将 PEU 基质植入大鼠皮下,以评估其引起炎症反应和促进血管生成的潜力。最后,使用临界大小的腓骨缺损和牙周模型来评估电纺 PEU BM 与临床可用的 Cytoflex 合成膜相比的再生能力。PEU BM 与 Cytoflex 具有相同的生物相容性,但在强度和弹性方面具有更优越的机械性能。此外,14 天后,PEU BM 表现出更高的 BGLAP/osteocalcin 表达量和更优越的体内性能--随着时间的推移,炎症消失,CD31 和 VWF 表达量增加。当将 PEU BM 植入大鼠小腿的临界大小缺损处时,PEU BM 可促进骨的形成,并具有较高的成骨和血管生成标志物表达。此外,8 周后,在已建立的牙周模型中,我们的膜增强了牙槽骨和骨水泥的再生。我们证明了 PEU BM 具有良好的临床特性,包括机械稳定性、细胞相容性以及在体外和体内促进骨形成。这凸显了其在牙周和颅面骨缺损中用于 GBR 的适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


