Chiroptical detection and mutation analysis of cancer-associated extracellular vesicles using microfluidics with oriented chiral nanoparticles
IF 17.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cancer-cell-secreted small extracellular vesicles, known as exosomes, represent a rapidly emerging family of cancer biomarkers. However, the current protocols for exosome analysis require complex equipment and lengthy procedures, which prevents their broad utilization for cancer diagnosis. We have engineered plasmonic gold nanoparticles combining molecular and nanoscale chirality, and have demonstrated that such nanoparticles in self-assembled films in a microfluidic device can isolate and analyze exosomes directly from blood plasma due to marker-specific chiroptical responses and volumetric electromagnetic resonance. Cancer exosomes can be distinguished from those from healthy donors by their giant polarization rotation signatures, and the observed dependence of plasmonic resonances on mutations of epidermal growth factor receptor suggests the possibility of in-line mutation/deletion analysis of protein cargo based on molecular chirality. The present microfluidic chips eliminate ultracentrifugation and improve the sensitivity and detection speed by at least 14 times and 10 times, respectively, enabling the rapid liquid biopsy of cancer.

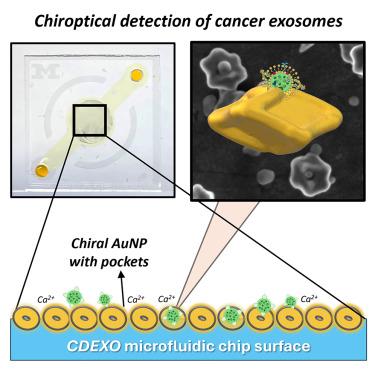
利用带有定向手性纳米粒子的微流体技术对癌症相关细胞外囊泡进行光学检测和突变分析
癌细胞分泌的小细胞外囊泡被称为外泌体,它代表了一个迅速崛起的癌症生物标志物家族。然而,目前的外泌体分析方案需要复杂的设备和冗长的程序,这阻碍了它们在癌症诊断中的广泛应用。我们设计了结合分子和纳米级手性的等离子体金纳米粒子,并在微流体设备中的自组装膜中证明了这种纳米粒子可以直接从血浆中分离和分析外泌体,因为它具有标记特异性的气光响应和体积电磁共振。癌症外泌体可通过其巨大的极化旋转特征与健康供体的外泌体区分开来,而观察到的等离子体共振与表皮生长因子受体突变的相关性表明,有可能根据分子手性对蛋白质货物进行在线突变/缺失分析。目前的微流控芯片无需超速离心,灵敏度和检测速度分别提高了至少 14 倍和 10 倍,从而实现了癌症的快速液体活检。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Matter
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
26.30
自引率
2.60%
发文量
367
期刊介绍:
Matter, a monthly journal affiliated with Cell, spans the broad field of materials science from nano to macro levels,covering fundamentals to applications. Embracing groundbreaking technologies,it includes full-length research articles,reviews, perspectives,previews, opinions, personnel stories, and general editorial content.
Matter aims to be the primary resource for researchers in academia and industry, inspiring the next generation of materials scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


