Active–Inactive Molten Salt Synthesis of Li- and Mn-Rich Layered Oxide Single Crystals as Cathode Materials for All-Solid-State Batteries
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
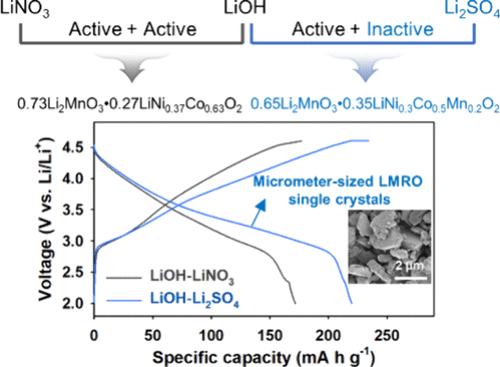
Micrometer-sized layered oxide single crystals are considered promising cathode materials for all-solid-state batteries (ASSBs) due to their superior properties compared to those of polycrystalline forms. In addition, Li- and Mn-rich layered oxides (LMRO), represented by the formula xLi2MnO3·(1–x)LiTMO2 (where TM = Ni, Co, and Mn), are noted for their higher energy densities and cost-effectiveness relative to conventional layered LiNi1–y–zCoyMnzO2 materials. In this regard, micrometer-sized, monodisperse, and discrete LMRO single crystals are synthesized using an active–inactive molten salt method that exploits the high reactivity of LiOH and the negligible reactivity of Li2SO4. This approach overcomes the challenges associated with conventional molten salt synthesis in controlling the structure and composition of LMRO. The chemical reactivities of lithium salts (LiOH, LiNO3, and Li2SO4) with transition metal hydroxide precursors are examined to synthesize LMRO single crystals. Our findings show that LiOH and LiNO3 are highly reactive, whereas Li2SO4 remains significantly inert. For this reason, the active–active LiOH–LiNO3 system forms the LMRO structure (0.73Li2MnO3·0.27Li[Ni0.37Co0.63]O2) with Mn-free NCM domains, regardless of the molar fractions of LiOH in LiOH–LiNO3. In contrast, the active–inactive LiOH–Li2SO4 system undergoes significant transformations from spinel to layered structures upon variation of the molar fraction of LiOH. At a LiOH molar fraction of 0.82, this system ultimately forms monodisperse LMRO single crystals (0.65Li2MnO3·0.35Li[Ni0.3Co0.5Mn0.2]O2) with Mn-containing NCM domains. Moreover, LMRO single crystals are investigated as high-capacity cathode materials for ASSBs. In particular, LMRO single crystals (0.65Li2MnO3·0.35Li[Ni0.3Co0.5Mn0.2]O2) demonstrate excellent electrochemical performance in ASSBs, achieving high reversible capacities of 220 mA h g–1 and stable capacity retention over 300 cycles. These findings underscore the critical role of lithium salt reactivity in determining the structural and compositional characteristics of LMRO single crystals during synthesis, providing valuable insights for improving the electrochemical performance of high-capacity LMRO cathode materials in ASSBs.
活性-非活性熔盐合成富锂离子和锰离子层状氧化物单晶作为全固态电池的阴极材料
微米大小的层状氧化物单晶由于其优于多晶形式的性能,被认为是很有前途的全固态电池(ASSB)阴极材料。此外,以 xLi2MnO3-(1-x)LiTMO2(其中 TM = Ni、Co 和 Mn)公式表示的富锂离子和锰离子层状氧化物(LMRO),与传统的层状 LiNi1-y-zCoyMnzO2 材料相比,具有更高的能量密度和成本效益。在这方面,利用活性-活性熔盐法合成了微米级、单分散和离散的 LMRO 单晶体,该方法利用了 LiOH 的高反应性和 Li2SO4 的可忽略反应性。这种方法克服了传统熔盐合成法在控制 LMRO 结构和组成方面的难题。我们研究了锂盐(LiOH、LiNO3 和 Li2SO4)与过渡金属氢氧化物前驱体的化学反应性,以合成 LMRO 单晶。我们的研究结果表明,LiOH 和 LiNO3 具有很高的反应活性,而 Li2SO4 则保持明显的惰性。因此,无论 LiOH-LiNO3 中 LiOH 的摩尔分数是多少,活性-活性 LiOH-LiNO3 体系都能形成 LMRO 结构(0.73Li2MnO3-0.27Li[Ni0.37Co0.63]O2)和无锰的 NCM 结构域。相反,随着 LiOH 摩尔分数的变化,活性-非活性 LiOH-Li2SO4 体系会发生从尖晶石到层状结构的显著转变。当 LiOH 摩尔分数为 0.82 时,该体系最终形成单分散 LMRO 单晶体(0.65Li2MnO3-0.35Li[Ni0.3Co0.5Mn0.2]O2),并具有含 Mn 的 NCM 域。此外,还对 LMRO 单晶作为 ASSB 的高容量阴极材料进行了研究。特别是,LMRO 单晶体(0.65Li2MnO3-0.35Li[Ni0.3Co0.5Mn0.2]O2)在 ASSB 中表现出优异的电化学性能,实现了 220 mA h g-1 的高可逆容量和 300 个循环的稳定容量保持。这些发现强调了锂盐反应性在合成过程中决定 LMRO 单晶的结构和组成特性的关键作用,为提高 ASSB 中高容量 LMRO 正极材料的电化学性能提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


