Modular alkene synthesis from carboxylic acids, alcohols and alkanes via integrated photocatalysis
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Alkenes serve as versatile building blocks in diverse organic transformations. Despite notable advancements in olefination methods, a general strategy for the direct conversion of carboxylic acids, alcohols and alkanes into alkenes remains a formidable challenge owing to their inherent reactivity disparities. Here we demonstrate an integrated photochemical strategy that facilitates a one-pot conversion of these fundamental building blocks into alkenes through a sequential C(sp3)–C(sp3) bond formation–fragmentation process, utilizing an easily accessible and recyclable phenyl vinyl ketone as the ‘olefination reagent’. This practical method not only offers an unparalleled paradigm for accessing value-added alkenes from abundant and inexpensive starting materials but also showcases its versatility through various complex scenarios, including late-stage on-demand olefination of multifunctional molecules, chain homologation of acids and concise syntheses of bioactive molecules. Moreover, initiating from carboxylic acids, alcohols and alkanes, this protocol presents a complementary approach to traditional olefination methods, making it a highly valuable addition to the research toolkit for alkene synthesis. The synthesis of alkenes from carboxylic acids, alcohols and alkanes is a formidable challenge owing to their inherent differences in reactivity. Now the one-pot conversion of these building blocks into alkenes is reported through an integrated photochemical strategy using a phenyl vinyl ketone as the olefination reagent.
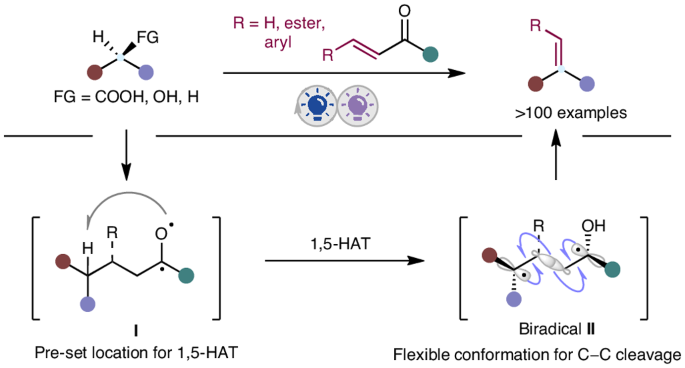
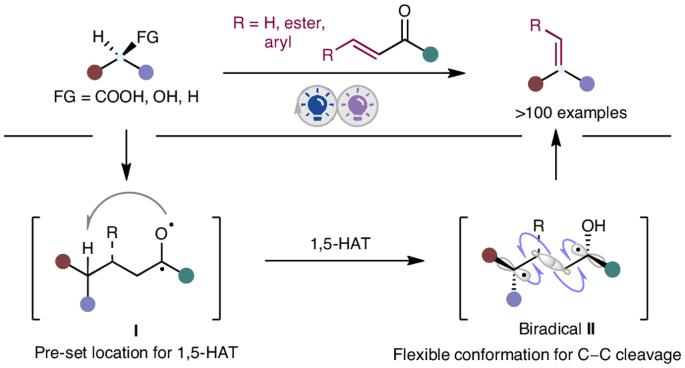
通过集成光催化技术从羧酸、醇和烷烃合成模块化烯烃
在各种有机转化过程中,烯烃是用途广泛的构建基块。尽管烯化方法取得了显著进步,但由于羧酸、醇和烷固有的反应性差异,将它们直接转化为烯的通用策略仍然是一项艰巨的挑战。在这里,我们展示了一种综合光化学策略,利用一种容易获得且可回收的苯基乙烯基酮作为 "烯化试剂",通过一个顺序的 C(sp3)-C(sp3)键形成-断裂过程,促进这些基本构件向烯的一锅式转化。这种实用的方法不仅为从丰富而廉价的起始材料中获得高附加值烯烃提供了一个无与伦比的范例,而且还通过各种复杂的情况展示了其多功能性,包括多功能分子的后期按需烯化、酸链同源化和生物活性分子的简易合成。此外,该方案以羧酸、醇和烷烃为起始原料,是对传统烯烃化方法的一种补充,使其成为烯烃合成研究工具包中极具价值的补充。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


