A Bi–Cu bimetallene array/carbonic anhydrase biohybrid for efficient and selective CO2 electroreduction at low concentration
IF 10.7
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The dramatic increase in CO2 emission has caused extreme weather events in recent years. Electrocatalytic CO2 reduction reaction (CO2RR) to useful fuels is an effective way of solving CO2 emission. However, serious hydrogen reaction evolution interference and low Faraday efficiency restrict its large-scale application, especially at low CO2 concentrations. This study presents a novel biohybrid comprising Bi–Cu bimetallenes (Bi–Cu BMLs) and carbonic anhydrase (CA) for efficient and selective electroreduction of CO2 to formic acid at low CO2 concentration. Ultra-thin Bi–Cu BMLs were synthesized via a facile galvanic replacement reaction, providing abundant sites for CA immobilization. The incorporation of Bi effectively suppresses the hydrogen evolution reaction and enhances the selectivity of the formic acid product, while the immobilized CA significantly increases the local CO2 concentration at the electrode surface due to its exceptional CO2 hydration activity and rapidly reversible equilibrium. As a result, the CA/Bi–Cu BML biohybrid system demonstrates remarkable performance, achieving 100% selectivity and 88.57% faradaic efficiency for formic acid production. Notably, the system maintains a high faradaic efficiency of 77.58% even at 5% CO2 concentration. Furthermore, the biohybrid catalyst exhibits excellent stability and reusability, underscoring its potential for practical applications in dilute CO2 streams.
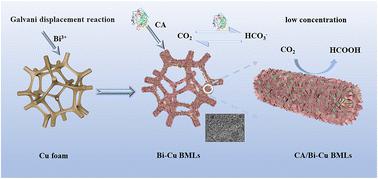
用于低浓度下高效和选择性二氧化碳电还原的双铜双金属阵列/碳酸酐酶生物混合物
近年来,二氧化碳排放量的急剧增加引发了极端天气事件。电催化二氧化碳还原反应(CO2RR)生成有用燃料是解决二氧化碳排放问题的有效途径。然而,严重的氢反应进化干扰和较低的法拉第效率限制了其大规模应用,尤其是在二氧化碳浓度较低的情况下。本研究提出了一种由双铜双金属(Bi-Cu BMLs)和碳酸酐酶(CA)组成的新型生物混合体,用于在低二氧化碳浓度下高效、选择性地将二氧化碳电还原成甲酸。超薄的 Bi-Cu BMLs 是通过简单的电化学置换反应合成的,为固定 CA 提供了丰富的位点。Bi 的加入有效抑制了氢进化反应并提高了甲酸产物的选择性,而固定的 CA 由于其优异的 CO2 水合活性和快速可逆平衡,显著提高了电极表面的局部 CO2 浓度。因此,CA/Bi-Cu BML 生物杂交系统表现出卓越的性能,甲酸生产的选择性达到 100%,远红外效率达到 88.57%。值得注意的是,即使在二氧化碳浓度为 5%的情况下,该系统也能保持 77.58% 的高远缘效率。此外,该生物杂化催化剂还表现出卓越的稳定性和可重复使用性,凸显了其在稀释二氧化碳流中的实际应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


