Synthesis, cytotoxicity and molecular docking of novel quinazoline-4(3H)-thione derivatives as EGFR-TKIs
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Quinazoline nucleus is a distinct scaffold with fascinating pharmacological characteristics. New quinazoline-4(3H)-thiones were synthesized and evaluated for their cytotoxic properties against two human cancer cell lines. Molecular investigations on their mechanism of action were conducted. The most potent derivatives were examined by molecular docking as EGFR-TKIs, and their ADME properties were predicted using the SwissADME tool. Derivatives 4, 10, and 12 exhibited the most notable cytotoxicity, as evidenced by their ability to upregulate p53 and caspase-3 expression while downregulating CDK1, inhibiting EGFR activity and downregulating EGFR and ERK1/2 signaling. These derivatives docked well with EGFR and had promising drug-like properties. Our derivatives deserve further optimization not only as novel anticancer agents but also as potent EGFR-TKIs.
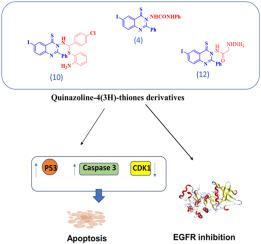
新型喹唑啉-4(3H)-硫酮衍生物作为表皮生长因子受体-TKIs的合成、细胞毒性和分子对接
喹唑啉核是一种独特的支架,具有迷人的药理特性。我们合成了新的喹唑啉-4(3H)-硫酮,并评估了它们对两种人类癌细胞系的细胞毒性。对它们的作用机制进行了分子研究。作为表皮生长因子受体抑制剂(EGFR-TKIs),对最有效的衍生物进行了分子对接研究,并使用 SwissADME 工具预测了它们的 ADME 特性。衍生物 4、10 和 12 表现出了最显著的细胞毒性,这体现在它们能够上调 p53 和 caspase-3 的表达,同时下调 CDK1、抑制表皮生长因子受体的活性以及下调表皮生长因子受体和 ERK1/2 的信号转导。这些衍生物与表皮生长因子受体对接良好,具有类似药物的特性。我们的衍生物不仅可以作为新型抗癌剂,还可以作为有效的表皮生长因子受体抑制剂(EGFR-TKIs),值得进一步优化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


