Copper(II) and Zinc(II) complexes of new water-soluble Schiff base ligands and their antiproliferative properties towards mesothelioma cell line
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-09-24
DOI:10.1016/j.jphotochem.2024.116049
引用次数: 0
Abstract
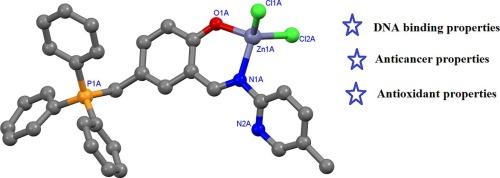
In this work, three Schiff base ligands (HL1-HL3) containing a triphenyl phosphonium cation and their Cu(II) and Zn(II) complexes with the general formulae of [M(L)Cl2] were synthesized and their structures were characterized by spectroscopic and analytical methods. Crystal structure of complex [Zn(L1)Cl2] was determined. In the structure of the complex, each Zn(II) ion is four coordinated binding to phenolate oxygen and imine nitrogen atoms of the ligand L1 and two chloride atoms in approximately tetrahedral geometry. The equilibrium geometry of three Schiff base ligands (HL1-HL3) and their Cu(II) and Zn(II) complexes were calculated using the density functional theory (DFT/B3LYP) method with the 6–31++G(d,p) basis set for the C, H, Cl, N, O, P atoms and LANL2DZ for the I atom. Frontier molecular orbitals (HOMO, LUMO) analyses were conducted for the optimized geometries of the compounds, and chemical reactivity descriptors such as hardness, softness, electrophilicity, and electronegativity were examined. Additionally, the molecular electrostatic potential (MEP) of all compounds was modeled using DFT calculations. These calculations were thoroughly examined, and their implications were discussed. The ligands and their metal complexes were investigated for their DNA binding properties. The compounds showed comparable DNA binding properties to ethidium bromide (EB) and 5-fluorouracyl (5-Fu) with DNA binding constant (Kb) 1.5–6.25 × 105 M−1. The cytotoxic properties of the compounds towards HUVEC and H2452 (mesothelioma cell line) cells were investigated using an MTS assay. The IC50 values determined for HUVEC cells were found to range between 27.28 µg/mL ([Cu(L2)Cl2]) and 273.1 µg/mL ([Zn(L2)Cl2]), while in H2452 cells, they varied from 39.48 µg/mL ([Cu(L2)Cl2]) to 319.3 µg/mL ([Zn(L3)Cl2]). The compounds HL1, HL3, and [Zn(L3)Cl2] exhibited antioxidant activity at the highest concentration (750 µg/mL) only, while [Zn(L1)Cl2], HL2, [Zn(L2)Cl2], [Cu(L2)Cl2], and [Cu(L3)Cl2] showed antioxidant activity at 250 µg/mL.
新型水溶性希夫碱配体的铜(II)和锌(II)配合物及其对间皮瘤细胞系的抗增殖特性
本研究合成了三种含有三苯基鏻阳离子的希夫碱配体(HL1-HL3)及其通式为[M(L)Cl2]的铜(II)和锌(II)配合物,并通过光谱和分析方法对它们的结构进行了表征。确定了[Zn(L1)Cl2]配合物的晶体结构。在该配合物的结构中,每个 Zn(II) 离子与配体 L1 的苯酚氧原子和亚胺氮原子以及两个氯原子呈近似四面体的四配位结合。使用密度泛函理论(DFT/B3LYP)方法计算了三种希夫碱配体(HL1-HL3)及其铜(II)和锌(II)配合物的平衡几何形状,其中 C、H、Cl、N、O、P 原子采用 6-31++G(d,p) 基,I 原子采用 LANL2DZ 基。对化合物的优化几何结构进行了前沿分子轨道(HOMO、LUMO)分析,并考察了化学反应性描述指标,如硬度、软度、亲电性和电负性。此外,还利用 DFT 计算对所有化合物的分子静电势(MEP)进行了建模。对这些计算进行了深入研究,并讨论了它们的影响。对配体及其金属复合物的 DNA 结合特性进行了研究。这些化合物的 DNA 结合性能与溴化乙锭(EB)和 5-氟尿嘧啶(5-Fu)相当,DNA 结合常数(Kb)为 1.5-6.25 × 105 M-1。化合物对 HUVEC 和 H2452(间皮瘤细胞系)细胞的细胞毒性采用 MTS 法进行了研究。对 HUVEC 细胞测定的 IC50 值介于 27.28 µg/mL ([Cu(L2)Cl2])和 273.1 µg/mL ([Zn(L2)Cl2])之间,而对 H2452 细胞测定的 IC50 值介于 39.48 µg/mL ([Cu(L2)Cl2])和 319.3 µg/mL ([Zn(L3)Cl2])之间。化合物 HL1、HL3 和[Zn(L3)Cl2]仅在最高浓度(750 微克/毫升)时显示出抗氧化活性,而[Zn(L1)Cl2]、HL2、[Zn(L2)Cl2]、[Cu(L2)Cl2]和[Cu(L3)Cl2]在 250 微克/毫升时显示出抗氧化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


