Maeruines A−E, elusive indole alkaloids from stems of Maerua siamensis and their inhibitory effects on cyclooxygenases and HT-29 colorectal cancer cell proliferation
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
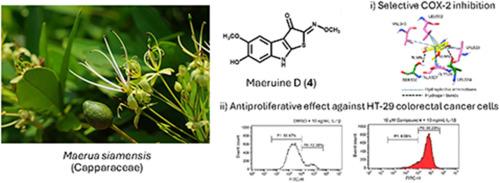
Five previously undescribed indole alkaloids, maeruines A−E (1−5), bearing imino-2H-thieno[2,3-b]indol-3(8H)-one skeleton, were obtained from the stems of Maerua siamensis. Their chemical structures were elucidated using spectroscopic techniques [NMR, MS, IR, and UV], and single-crystal X-ray diffraction. Maeruine D (4) displayed selective cyclooxygenase-2 (COX-2) inhibitory activity in vitro with an IC50 of 29.72 ± 6.36 μM. Molecular dynamics simulations revealed that maeruine D could form a stable complex with human COX-2, predominantly driven by hydrophobic interactions. In addition, five amino-acid residues including Val349, Leu352, Leu384, Val523, and Ala527 were identified as hot-spot ones, which may lead to high binding affinity and selectivity. Furthermore, it exhibited cytotoxicity against HT-29 colorectal cancer cells with an IC50 of 29.32 ± 4.76 μM, and, at 0.1−10 μM, significantly inhibited their proliferation, induced by the proinflammatory cytokine interleukin-1β (IL-1β), in a dose-dependent manner.
茜草茎中难以捉摸的吲哚生物碱 Maeruines A-E 及其对环氧化酶和 HT-29 大肠癌细胞增殖的抑制作用
从暹罗缅菊的茎中获得了五种以前未曾描述过的吲哚生物碱--缅菊碱 A-E(1-5),它们具有亚氨基-2H-噻吩并[2,3-b]吲哚-3(8H)-酮骨架。利用光谱技术[核磁共振、质谱、红外光谱和紫外光谱]和单晶 X 射线衍射法阐明了它们的化学结构。Maeruine D(4)在体外显示出选择性环氧化酶-2(COX-2)抑制活性,其 IC50 为 29.72 ± 6.36 μM。分子动力学模拟显示,maeruine D 能与人 COX-2 形成稳定的复合物,主要是由疏水相互作用驱动的。此外,包括 Val349、Leu352、Leu384、Val523 和 Ala527 在内的五个氨基酸残基被确定为热点残基,这可能会导致高结合亲和力和选择性。此外,它对 HT-29 大肠癌细胞具有细胞毒性,IC50 为 29.32 ± 4.76 μM,并且在 0.1-10 μM 的剂量下,能显著抑制由促炎细胞因子白细胞介素-1β(IL-1β)诱导的细胞增殖,且呈剂量依赖性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


