A robust shape model for blood vessels analysis
IF 4.3
3区 材料科学
Q1 ENGINEERING, ELECTRICAL & ELECTRONIC
引用次数: 0
Abstract
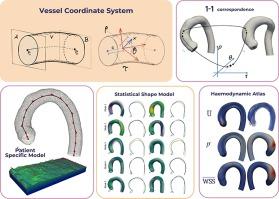
The availability of digital twins for the cardiovascular system will enable insightful computational tools both for research and clinical practice. This, however, demands robust and well defined models and methods for the different steps involved in the process. We present a vessel coordinate system (VCS) that enables the unambiguous definition of locations in a vessel section, by adapting the idea of cylindrical coordinates to the vessel geometry. Using the VCS model, point correspondence can be defined among different samples of a cohort, allowing data transfer, quantitative comparison, shape coregistration or population analysis. Furthermore, the VCS model allows for the generation of specific meshes (e.g. cylindrical grids, OGrids) necessary for an accurate reconstruction of the geometries used in fluid simulations. We provide the technical details for coordinates computation and discuss the assumptions taken to guarantee that they are well defined. The VCS model is tested in a series of applications. We present a robust, low dimensional, patient specific vascular model and use it to study phenotype variability analysis of the thoracic aorta within a cohort of patients. Point correspondence is exploited to build an haemodynamics atlas of the aorta for the same cohort. The atlas originates from fluid simulations (Navier-Stokes with Finite Volume Method) conducted using OpenFOAMv10. We finally present a relevant discussion on the VCS model, which covers its impact in important areas such as shape modeling and computer fluids dynamics (CFD).
用于血管分析的稳健形状模型
心血管系统数字双胞胎的出现,将为研究和临床实践提供具有洞察力的计算工具。然而,这就要求对这一过程中涉及的不同步骤采用稳健、定义明确的模型和方法。我们提出了一种血管坐标系(VCS),通过将圆柱坐标的概念应用于血管几何,该坐标系能够明确定义血管截面中的位置。使用血管坐标系模型,可以在队列的不同样本之间定义点对应关系,从而进行数据传输、定量比较、形状核心注册或群体分析。此外,VCS 模型还可以生成特定的网格(如圆柱网格、OGrids),这些网格是精确重建流体模拟中使用的几何图形所必需的。我们提供了坐标计算的技术细节,并讨论了保证坐标定义明确的假设条件。我们在一系列应用中对 VCS 模型进行了测试。我们提出了一个稳健、低维、针对特定患者的血管模型,并将其用于研究一组患者胸主动脉的表型变异性分析。利用点对应关系,为同一队列的患者建立主动脉血流动力学图谱。该图集源于使用 OpenFOAMv10 进行的流体模拟(纳维-斯托克斯有限体积法)。 最后,我们对 VCS 模型进行了相关讨论,涵盖了其在形状建模和计算机流体动力学(CFD)等重要领域的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


