Organocatalysts for L-Lactide polymerization: 2-alkyl- and 2-aryl-1,1,3,3-tetramethylguanidines
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A series of 2-alkyl- and 2-aryl-1,1,3,3-tetramethylguanidine derivatives were synthesized, and their application in L-Lactide (LA) polymerization was studied. Bn-TMG was the best catalyst in LA polymerization ( [Bn-TMG] = 5 mM, conversion = 94 % after 4 h at 25 °C) compared to other monoguanidine derivatives. In addition, the catalytic activities of C2-dTMG exhibited higher catalytic activity than Bn-TMG, nBu-TMG (monoguanidine derivatives), and C3-dTMG (diguanidine derivative). Density functional theory calculations revealed that Bn-TMG deprotonated benzyl alcohol enhanced benzyl alcohol's initiation ability to LA, and the protonated Bn-TMG activated LA ring-opening process by interacting with the ether oxygen atom of LA. Bn-TMG presented excellent catalytic activity and controllability for LA polymerization at 25 °C.
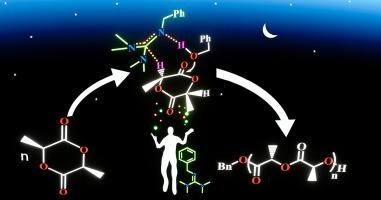
用于 L-内酯聚合的有机催化剂:2-烷基和 2-芳基-1,1,3,3-四甲基胍
研究人员合成了一系列 2-烷基和 2-芳基-1,1,3,3-四甲基胍衍生物,并研究了它们在 L-内酰胺(LA)聚合中的应用。与其他单胍衍生物相比,Bn-TMG 是 LA 聚合过程中的最佳催化剂([Bn-TMG] = 5 mM,25 °C 下 4 小时后转化率 = 94 %)。此外,C2-dTMG 的催化活性高于 Bn-TMG、nBu-TMG(单胍衍生物)和 C3-dTMG(二胍衍生物)。密度泛函理论计算表明,Bn-TMG 对苯甲醇的去质子化作用增强了苯甲醇对 LA 的引发能力,质子化的 Bn-TMG 通过与 LA 的醚氧原子相互作用激活了 LA 的开环过程。Bn-TMG 在 25 °C 下对 LA 聚合具有优异的催化活性和可控性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


