Dopamine-mediated formation of a memory module in the nucleus accumbens for goal-directed navigation
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
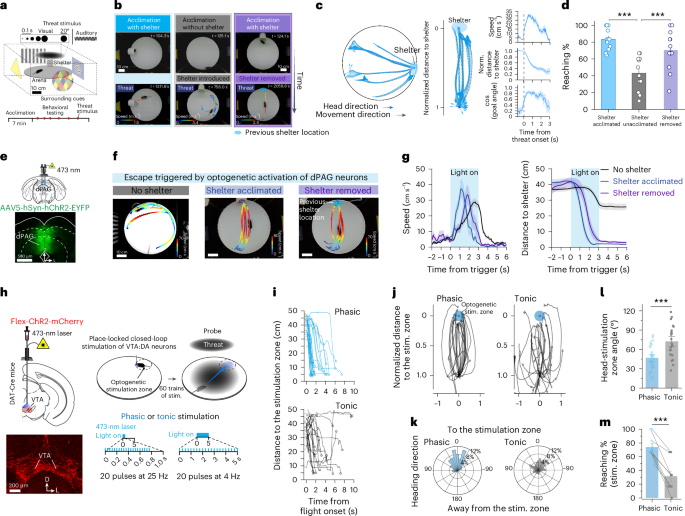
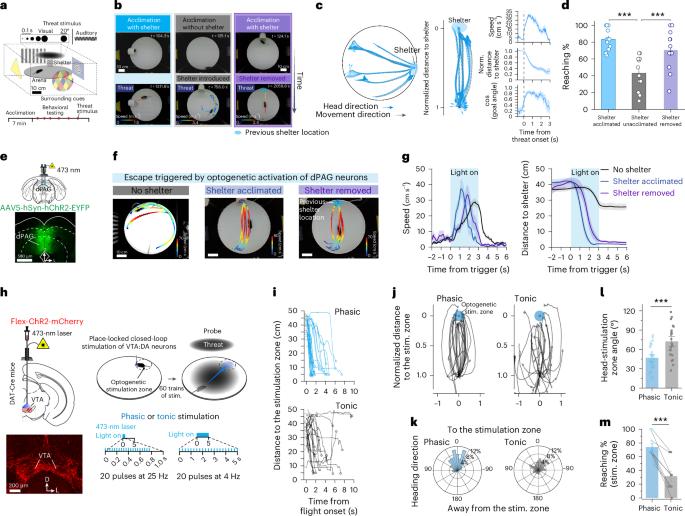
Spatial memories guide navigation efficiently toward desired destinations. However, the neuronal and circuit mechanisms underlying the encoding of goal locations and its translation into goal-directed navigation remain unclear. Here we demonstrate that mice rapidly form a spatial memory of a shelter during shelter experiences, guiding escape behavior toward the goal location—a shelter—when under threat. Dopaminergic neurons in the ventral tegmental area and their projection to the nucleus accumbens (NAc) encode safety signals associated with the shelter. Optogenetically induced phasic dopamine signals are sufficient to create a place memory that directs escape navigation. Converging dopaminergic and hippocampal glutamatergic inputs to the NAc mediate the formation of a goal-related memory within a subpopulation of NAc neurons during shelter experiences. Artificial co-activation of this goal-related NAc ensemble with neurons in the dorsal periaqueductal gray was sufficient to trigger memory-guided, rather than random, escape behavior. These findings provide causal evidence of cognitive circuit modules linking memory with goal-directed action. Jung et al. show that shelter experience boosts dopamine release in the nucleus accumbens, generating a goal-location memory. Reactivating a neuronal ensemble developed from shelter experience enables memory-guided navigation to the goal during escape.
多巴胺介导的目标定向导航核记忆模块的形成
空间记忆能引导导航有效地到达期望的目的地。然而,目标位置编码及其转化为目标导航的神经元和电路机制仍不清楚。在这里,我们证明了小鼠在避难所经历中会迅速形成避难所的空间记忆,从而在受到威胁时引导小鼠向目标地点--避难所--逃逸。小鼠腹侧被盖区的多巴胺能神经元及其向脑下垂核(NAc)的投射编码了与庇护所相关的安全信号。光遗传诱导的阶段性多巴胺信号足以产生地方记忆,从而指导逃生导航。多巴胺能和海马谷氨酸能汇聚到NAc的输入介导了NAc神经元亚群在避难所经历中目标相关记忆的形成。人为地将这一与目标相关的 NAc 神经元集合与背侧下uctal 灰色神经元共同激活,足以触发记忆引导的而非随机的逃逸行为。这些发现提供了将记忆与目标引导的行为联系起来的认知回路模块的因果证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


