Diverse Effects of SO2-Induced Pt–O–SO3 on the Catalytic Oxidation of C3H6 and C3H8
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The effects of sulfur dioxide (SO2) in the catalytic purification of short-chain hydrocarbons are still controversial, and the exact role of SO2 on adsorption and reaction pathways during the catalytic oxidation of different volatile organic compounds (VOCs) remains unclear. Herein, a three-dimensional ordered macroporous Ce0.8Zr0.2O2 supported Pt nanoparticle monolithic catalyst (Pt/OM CZO) was synthesized to investigate these effects. Our findings uncover the diverse effects of SO2: Upon SO2 treatment, the coupling between the S 3p and Pt 5d orbitals promotes the Pt–O–SO3 structure in situ formed on the catalyst surface. The propene (C3H6) molecule readily binds with the oxygen atom in Pt–O–SO3, resulting in the accumulation of acetone and carbon deposition, thereby hindering C3H6 oxidation. Conversely, a cleaved oxygen atom within the Pt–O–SO3 structure enhances propane (C3H8) adsorption and activates the C–H bond, facilitating C3H8 oxidation. These insights are pivotal for advancing the frontier of sulfur-tolerant catalysts, addressing both economic and environmental challenges.
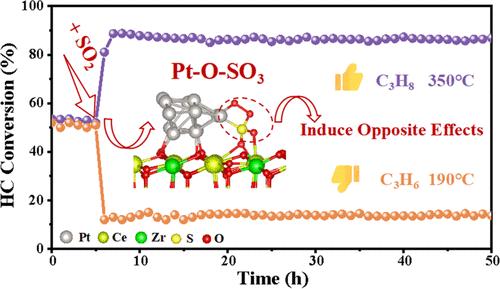
二氧化硫诱导的 Pt-O-SO3 对 C3H6 和 C3H8 催化氧化的不同影响
二氧化硫(SO2)对短链烃类催化净化的影响仍存在争议,SO2 在不同挥发性有机化合物(VOC)催化氧化过程中对吸附和反应途径的确切作用仍不清楚。在此,我们合成了一种三维有序大孔 Ce0.8Zr0.2O2 支承铂纳米颗粒整体催化剂(Pt/OM CZO)来研究这些影响。我们的研究结果揭示了二氧化硫的多种效应:经二氧化硫处理后,S 3p 和 Pt 5d 轨道之间的耦合促进了催化剂表面原位形成 Pt-O-SO3 结构。丙烯(C3H6)分子很容易与 Pt-O-SO3 中的氧原子结合,导致丙酮积累和碳沉积,从而阻碍了 C3H6 的氧化。相反,Pt-O-SO3 结构中的氧原子裂解会增强对丙烷(C3H8)的吸附并激活 C-H 键,从而促进 C3H8 的氧化。这些见解对于推动耐硫催化剂的发展、应对经济和环境挑战至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


