VUV Activated Fe(VI) by Promoting the Generation of Intermediate Valent Iron and Hydroxyl Radicals
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
In this study, vacuum ultraviolet (VUV) was first proposed to activate ferrate (Fe(VI)) for degrading micropollutants (e.g., carbamazepine (CBZ)). Results indicated that VUV/Fe(VI) could significantly facilitate the CBZ degradation, and the removal efficiencies of VUV/Fe(VI) were 30.9–83.4% higher than those of Fe(VI) at pH = 7.0–9.0. Correspondingly, the degradation rate constants of VUV/Fe(VI) were 2.3–36.0-fold faster than those of Fe(VI). Free radical quenching and probe experiments revealed that the dominant active species of VUV/Fe(VI) were •OH and Fe(V)/Fe(IV), whose contribution ratios were 43.3 to 48.6% and 48.2 to 46.6%, respectively, at pH = 7.0–9.0. VUV combined with Fe(VI) not only effectively mitigated the weak oxidizing ability of Fe(VI) under alkaline conditions (especially pH = 9.0) but also attenuated the deteriorating effect of background constituents on Fe(VI). In different real waters (tap water, river water, WWTPs effluent), VUV/Fe(VI) retained a remarkably enhanced effect on CBZ degradation compared to Fe(VI). Moreover, VUV/Fe(VI) exhibited outstanding performance in the debasement of CBZ and sulfamethoxazole (SMX), as well as six other micropollutants, displaying broad-spectrum capability in degrading micropollutants. Overall, this study developed a novel oxidation process that was efficient and energy-saving for the rapid removal of micropollutants.
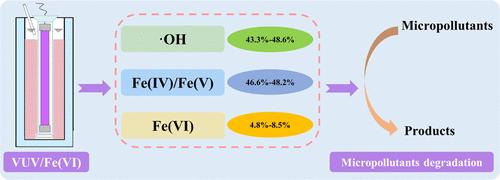
紫外线通过促进生成中间价铁和羟基自由基活化铁(VI)
本研究首次提出用真空紫外线(VUV)活化铁(Fe(VI))来降解微污染物(如卡马西平(CBZ))。结果表明,紫外/铁(VI)能显著促进 CBZ 的降解,在 pH = 7.0-9.0 的条件下,紫外/铁(VI)的去除率比铁(VI)高 30.9-83.4%。相应地,紫外/铁(VI)的降解速率常数比铁(VI)快 2.3-36.0 倍。自由基淬灭和探针实验表明,在 pH = 7.0-9.0 条件下,紫外/铁(VI)的主要活性物种是-OH 和铁(V)/铁(IV),其贡献率分别为 43.3% 至 48.6%,48.2% 至 46.6%。紫外线与铁(VI)的结合不仅有效地缓解了铁(VI)在碱性条件下(尤其是 pH = 9.0)的弱氧化能力,还削弱了背景成分对铁(VI)的恶化作用。在不同的实际水体(自来水、河水、污水处理厂出水)中,紫外可见光/铁(VI)与铁(VI)相比,对 CBZ 降解的作用明显增强。此外,紫外/铁(VI)在降解 CBZ 和磺胺甲恶唑(SMX)以及其他六种微污染物方面表现突出,显示出广谱降解微污染物的能力。总之,这项研究开发出了一种新型氧化工艺,既高效又节能,可快速去除微污染物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


