A Novel Ce─Mn Heterojunction-Based Multi-Enzymatic Nanozyme with Cancer-Specific Enzymatic Activity and Photothermal Capacity for Efficient Tumor Combination Therapy
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Catalytic medicine, using enzymes or nanozymes, is an emerging method for cancer treatment. However, its applicability is limited by the low catalytic activity in the tumor microenvironment (TME). In this work, a versatile and synthesis-friendly nanozyme, CeO2Mn1.08Ox nanoclusters, is prepared. This novel Ce─Mn heterojunction is formed by oxidation of CeO2 nanoparticles through H2SO4/KMnO4. CeO2Mn1.08Ox exhibits high multi-enzymatic catalytic activities and acts as a catalase (CAT), peroxidase (POD), and oxidase (OXD) mimics under acidic conditions. It can regulate the TME by relieving hypoxia and consuming endogenous glutathione (GSH). Glucose oxidase (GOx) is then incorporated into CeO2Mn1.08Ox and linked with poly(ethylene glycol) (PEG) to obtain the cascade enzyme system (Ce─Mn)-PEI/GOx-PEG. CeO2Mn1.08Ox exhibits CAT-like properties, which sensitize GOx-based starvation therapy, and POD- and OXD-like properties, which generate highly cytotoxic reactive oxygen species (ROS) in cancer cells. The glucose catabolic product, H2O2, is also used to generate O2 and ROS. In addition, the heterojunction structure provides CeO2Mn1.08Ox with near-infrared (NIR) photothermal capability, making it suitable for photothermal therapy (PTT). Density functional theory (DFT) calculations provide possible reasons for the high catalytic activity and photothermal capability of CeO2Mn1.08Ox. When combining mild PTT with catalytic therapy, the cascade enzyme system (Ce─Mn)-PEI/GOx-PEG can efficiently ablate tumors.
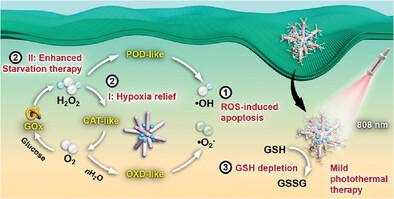
一种新型 Ce─Mn 异质结多酶纳米酶,具有癌症特异性酶活性和光热能力,可用于高效肿瘤联合疗法
使用酶或纳米酶的催化医学是一种新兴的癌症治疗方法。然而,由于其在肿瘤微环境(TME)中的催化活性较低,其适用性受到了限制。本研究制备了一种多功能且易于合成的纳米酶--CeO2Mn1.08Ox 纳米团簇。这种新型 Ce─Mn 异质结是通过 H2SO4/KMnO4 氧化 CeO2 纳米颗粒形成的。CeO2Mn1.08Ox 具有很高的多酶催化活性,在酸性条件下可作为过氧化氢酶(CAT)、过氧化物酶(POD)和氧化酶(OXD)的模拟物。它可以通过缓解缺氧和消耗内源性谷胱甘肽(GSH)来调节 TME。然后将葡萄糖氧化酶(GOx)纳入 CeO2Mn1.08Ox 并与聚乙二醇(PEG)连接,从而获得级联酶系统(Ce─Mn)-PEI/GOx-PEG。CeO2Mn1.08Ox 具有类似于 CAT 的特性,能敏化基于 GOx 的饥饿疗法;还具有类似于 POD 和 OXD 的特性,能在癌细胞中产生具有高度细胞毒性的活性氧(ROS)。葡萄糖分解产物 H2O2 也可用于产生 O2 和 ROS。此外,异质结结构使 CeO2Mn1.08Ox 具有近红外(NIR)光热能力,使其适用于光热疗法(PTT)。密度泛函理论(DFT)计算为 CeO2Mn1.08Ox 的高催化活性和光热能力提供了可能的原因。当温和的 PTT 与催化治疗相结合时,级联酶系统(Ce─Mn)-PEI/GOx-PEG 可以有效地消融肿瘤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


