Non-discriminating engineered masking of immuno-evasive ligands on tumour-derived extracellular vesicles enhances tumour vaccination outcomes
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
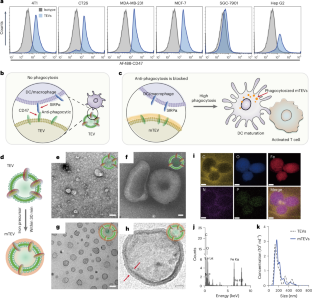
The success of personalized cancer immunotherapy depends on the initial tumour antigenic presentation to dendritic cells and macrophages. Tumour-derived extracellular vesicles (TEVs) contain abundant tumour antigenic molecules. The presence of anti-phagocytotic signals such as cluster of differentiation 47 (CD47) on the surface of the TEVs, however, leads to evasion of the same dendritic cells and macrophages. Here we show that iron oxide hydroxide nanocomposites can successfully mask TEV surfaces and unblock phagocytosis without affecting extracellular vesicles’ elicited immune goals. After internalization, the mask disintegrates in the lysosome, releasing the tumour antigenic cargo. This triggers antigen presentation and promotes dendritic cell activation and maturation and macrophage reprogramming in animal models, leading to a drastic reduction of tumour volume and metastasis, and in human malignant pleural effusion clinical samples. This straightforward masking strategy eliminates the ubiquitous anti-phagocytosis block found in clinical samples and can be applied universally across all patient-specific TEVs as tumour antigenic agents for enhanced immunotherapy. Nano-masking of the surface of tumour-derived extracellular vesicles blocks their immune-evasive action. This improves their phagocytosis by dendritic cells, leading to maturation of T-cell action and culminating in undampened anti-tumour immunity.

对肿瘤源性细胞外囊泡上的免疫侵袭配体进行非歧视性工程掩蔽可提高肿瘤疫苗接种效果
个性化癌症免疫疗法的成功取决于树突状细胞和巨噬细胞最初的肿瘤抗原呈递。肿瘤衍生的细胞外囊泡(TEV)含有丰富的肿瘤抗原分子。然而,TEVs 表面存在的抗吞噬信号(如分化簇 47(CD47))会导致树突状细胞和巨噬细胞逃避相同的抗吞噬信号。在这里,我们展示了氧化铁氢氧化物纳米复合材料可以成功地掩盖 TEV 表面,并在不影响细胞外囊泡诱导免疫目标的情况下解除吞噬作用。内化后,掩膜在溶酶体中分解,释放出肿瘤抗原货物。在动物模型和人类恶性胸腔积液临床样本中,这能触发抗原呈递,促进树突状细胞活化和成熟以及巨噬细胞重编程,从而大幅减少肿瘤体积和转移。这种直接的掩蔽策略消除了临床样本中无处不在的抗吞噬阻滞,可普遍应用于所有患者特异性 TEV,作为增强免疫疗法的肿瘤抗原制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


