Reactive Surface Explored by NAP-XPS: Why Ionic Conductors Are Promoters for Water Gas Shift Reaction
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
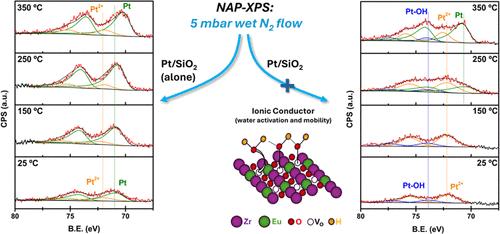
Near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) experiments have been carried out in N2 and N2–H2O atmospheres on a Pt-based catalyst physically mixed with an Eu-doped ZrO2 ionic conductor as a function of temperature under realistic conditions of the water gas shift (WGS) reaction. This work aims to demonstrate the significant effect of having active H2O on the ionic conductor surface at reaction temperatures to provide it to Pt metal sites. The ionic conductor, Eu-doped zirconia matrix, presents defects (oxygen vacancies, Ov) that allows upon H2O dissociation the formation of a hydrogen-bonded molecular water layer favoring diffusion through a Grotthuss mechanism below 300 °C. In the presence of H2O, the Ov are occupied by hydroxyl species as observed in the Eu 4d spectra, which differentiate two types of Eu oxidation states. The Eu3+-to-Eu2+ atomic ratio increases with the occupancy of the Ov by hydroxyls. Moreover, while the Pt-based catalyst alone is unable to create Pt–OH bonds, the physical mixture of the Pt-based catalyst and the ionic conductor allows the formation of Pt–OH bonds from room temperature up to 300 °C. These data demonstrate that the increase in molecular water concentration on the ionic conductor surface up to 300 °C acts as a reservoir to provide water to the Pt surface, enhancing the catalyst performance in the WGS reaction, supporting the importance of the surface H2O concentration in the reaction kinetics.
通过 NAP-XPS 探索反应表面:为什么离子导体是水气移反应的促进剂?
在水煤气变换(WGS)反应的实际条件下,在 N2 和 N2-H2O 大气中对与掺杂 Eu 的 ZrO2 离子导体物理混合的铂基催化剂进行了近常压 X 射线光电子能谱(NAP-XPS)实验。这项研究旨在证明在反应温度下离子导体表面的活性 H2O 对铂金属位点的重要影响。离子导体(掺杂 Eu 的氧化锆基体)存在缺陷(氧空位,Ov),因此在 H2O 解离后可形成氢键分子水层,有利于通过 Grotthuss 机制在 300 °C 以下进行扩散。在 H2O 存在的情况下,氧空位被羟基占据,如 Eu 4d 光谱所观察到的那样,从而区分出两种 Eu 氧化态。随着羟基占据 Ov,Eu3+-Eu2+ 原子比增加。此外,虽然单独的铂基催化剂无法形成 Pt-OH 键,但铂基催化剂和离子导体的物理混合物可在室温至 300 °C 的条件下形成 Pt-OH 键。这些数据表明,离子导体表面的分子水浓度在 300 ℃ 时的增加起到了向铂表面提供水分的蓄水池的作用,从而提高了催化剂在 WGS 反应中的性能,证明了表面 H2O 浓度在反应动力学中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


