Targeting the phosphatidylglycerol lipid: An amphiphilic dendrimer as a promising antibacterial candidate
IF 3.5
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The rapid emergence and spread of multidrug-resistant bacterial pathogens require the development of antibacterial agents that are robustly effective while inducing no toxicity or resistance development. In this context, we designed and synthesized amphiphilic dendrimers as antibacterial candidates. We report the promising potent antibacterial activity shown by the amphiphilic dendrimer AD1b, composed of a long hydrophobic alkyl chain and a tertiary amine-terminated poly(amidoamine) dendron, against a panel of Gram-negative bacteria, including multidrug-resistant Escherichia coli and Acinetobacter baumannii. AD1b exhibited effective activity against drug-resistant bacterial infections in vivo. Mechanistic studies revealed that AD1b targeted the membrane phospholipids phosphatidylglycerol (PG) and cardiolipin (CL), leading to the disruption of the bacterial membrane and proton motive force, metabolic disturbance, leakage of cellular components, and, ultimately, cell death. Together, AD1b that specifically interacts with PG/CL in bacterial membranes supports the use of small amphiphilic dendrimers as a promising strategy to target drug-resistant bacterial pathogens and addresses the global antibiotic crisis.
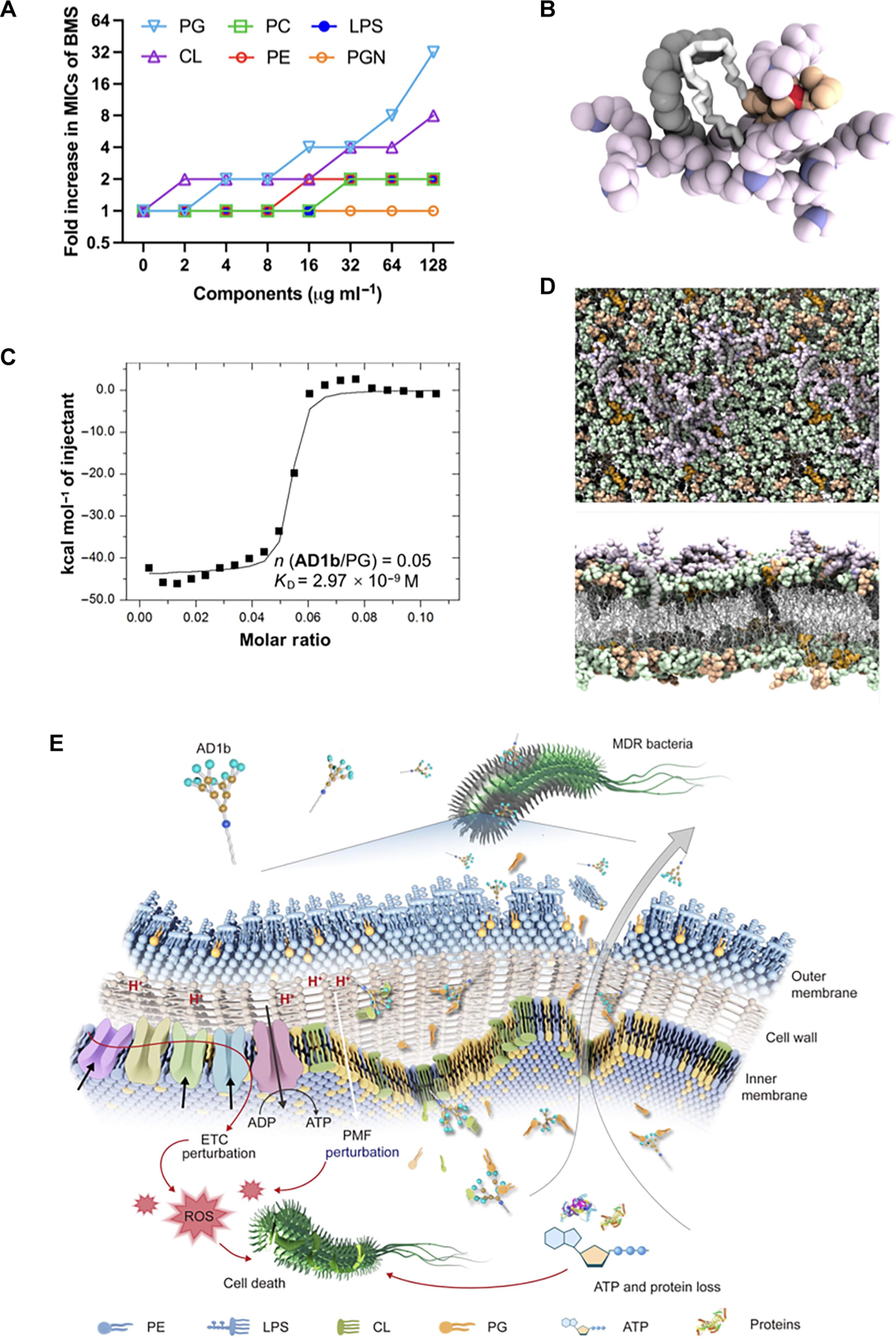
以磷脂酰甘油脂质为目标:两亲性树枝状聚合物有望成为抗菌候选物质
耐多药细菌病原体的迅速出现和传播要求开发出既能产生强效抗菌作用,又不会产生毒性或耐药性的抗菌剂。在此背景下,我们设计并合成了两亲性树枝状聚合物作为抗菌候选物。两亲性树枝状聚合物 AD1b 由长疏水烷基链和以叔胺为末端的聚(氨基胺)树枝状化合物组成,我们报告了 AD1b 对一系列革兰氏阴性菌(包括耐多药大肠杆菌和鲍曼不动杆菌)的抗菌活性。AD1b 对体内耐药细菌感染具有有效的活性。机理研究发现,AD1b 以膜磷脂磷脂酰甘油(PG)和心磷脂(CL)为靶标,导致细菌膜和质子动力破坏、代谢紊乱、细胞成分泄漏,最终导致细胞死亡。总之,能与细菌膜中的 PG/CL 发生特异性相互作用的 AD1b 支持使用小型两亲性树枝状聚合物作为针对耐药细菌病原体的一种有前途的策略,并能解决全球抗生素危机。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Chemical Biology
生物-生化与分子生物学
CiteScore
7.50
自引率
5.00%
发文量
353
审稿时长
3.3 months
期刊介绍:
ACS Chemical Biology provides an international forum for the rapid communication of research that broadly embraces the interface between chemistry and biology.
The journal also serves as a forum to facilitate the communication between biologists and chemists that will translate into new research opportunities and discoveries. Results will be published in which molecular reasoning has been used to probe questions through in vitro investigations, cell biological methods, or organismic studies.
We welcome mechanistic studies on proteins, nucleic acids, sugars, lipids, and nonbiological polymers. The journal serves a large scientific community, exploring cellular function from both chemical and biological perspectives. It is understood that submitted work is based upon original results and has not been published previously.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


