Selective adsorption of trace CO2 by immobilized amino acid ionic liquids with ultra-micropores based on amino MOFs
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Great attention has been paid to effectively capture trace CO2 in air or a confined space for ensuring human safety, but it remains challenging to enhance CO2 capacity and selectivity concurrently. In this study, metal–organic frameworks (MOFs) were combined with amino groups (NH2-MIL-125(Ti) and NH2-UIO-66(Zr)) and amino acid anion-functionalized ionic liquids (ILs), namely 1-Butyl-3-methylimidazolium glycinate ([BMIm]Gly) and 1-Butyl-3-methylimidazolium arginine ([BMIm]Arg) to prepare various ionic liquid (IL) composites containing various IL contents. Relative to pristine supports, incorporating ILs significantly enhanced CO2 capacity together with CO2/N2 selectivity, in particular in the confined spaces (<5000 ppm) or in air (415 ppm). At a 50 wt% IL loading, new ultra-micropores (<0.65 nm) could be obtained in IL-NH2-UIO-66(Zr) composite, but they did not occur within pristine supports or the rest IL-NH2-UIO-66(Zr) (10 wt%, 30 wt% and 70 wt%). Among them, CO2 uptake of 50 wt% [BMIm]Arg-NH2-UIO-66(Zr) peak at 0.0005 and 0.005 bar (2.93 and 3.87 mmolCO2/g-adsorbent, respectively) at 313 K; besides, recyclability significantly increased compared with the latest values reported. Additionally, the excellent optimal CO2/N2 selectivity was determined to be 8916 at 0.005 bar and 288 K, which increased by 254.7 folds relative to NH2-UIO-66(Zr). In the meantime, as revealed by mixed gas breakthrough experimental results, 50 wt% [BMIm]Arg-NH2-UIO-66(Zr) showed superb CO2 separation effect in air and simulated confined spaces. Such ultra-high CO2 separation efficacy is associated with the synergistic effect of chemical interaction of IL anion with amino groups in MOFs and CO2 and the novel ultra-micropore effect. Findings in the present work shed more lights on designing hierarchically porous IL composites that possess ultra-micropores to efficiently remove trace CO2.
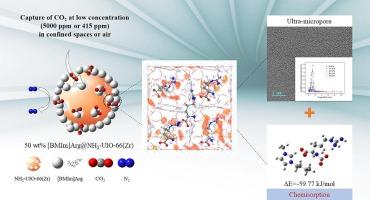
基于氨基 MOFs 的超微孔固定化氨基酸离子液体对痕量 CO2 的选择性吸附
为确保人类安全,有效捕获空气或密闭空间中的痕量二氧化碳已受到极大关注,但同时提高二氧化碳的容量和选择性仍具有挑战性。本研究将金属有机框架(MOFs)与氨基(NH2-MIL-125(Ti) 和 NH2-UIO-66(Zr))和氨基酸阴离子官能化离子液体(ILs)(即 1-丁基-3-甲基咪唑鎓甘氨酸盐([BMIm]Gly)和 1-丁基-3-甲基咪唑鎓精氨酸盐([BMIm]Arg))相结合,制备了含有不同 IL 含量的各种离子液体(IL)复合材料。与原始支撑物相比,IL 的加入显著提高了 CO2 容量和 CO2/N2 选择性,尤其是在密闭空间(<5000 ppm)或空气(415 ppm)中。当 IL 含量为 50 wt%时,IL-NH2-UIO-66(Zr)复合材料中会出现新的超微孔(<0.65 nm),但在原始支撑物或其余 IL-NH2-UIO-66(Zr) (10 wt%、30 wt% 和 70 wt%)中则不会出现。其中,50 wt%[BMIm]Arg-NH2-UIO-66(Zr)在 313 K 时的二氧化碳吸收峰值为 0.0005 和 0.005 bar(分别为 2.93 和 3.87 mmolCO2/g-吸附剂);此外,与最新报道的数值相比,可回收性显著提高。此外,在 0.005 巴和 288 K 条件下,最佳 CO2/N2 选择性为 8916,比 NH2-UIO-66(Zr)提高了 254.7 倍。同时,混合气体突破实验结果表明,50 wt% [BMIm]Arg-NH2-UIO-66(Zr) 在空气和模拟密闭空间中表现出卓越的二氧化碳分离效果。这种超高的二氧化碳分离效果与 IL 阴离子与 MOFs 中氨基的化学作用和二氧化碳的协同效应以及新型超微孔效应有关。本研究的发现为设计具有超微孔的分层多孔 IL 复合材料以高效去除痕量 CO2 提供了更多启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


