Chemoselective oxidative N-debenzylation of aryl halogenated amines using the Laccase LAC97/TEMPO system
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The N-debenzylation of aryl-halogenated amines using the Laccase LAC97/TEMPO system is reported, demonstrating selective N-debenzylation with no dehalogenation of the phenyl ring, which is observed with conventional debenzylation methods such as palladium-catalysed hydrogenation. This reaction was performed under ambient conditions in acidic buffer with 5 % DMSO as co-solvent in an open-to-air vessel. The versatility and robustness of the system was demonstrated on substrates harbouring different halogen moieties. The limitations of the Laccase LAC97/TEMPO system were also studied, highlighting the formation of by-products due to overoxidation on selected substrates. A series of substrates with various halogens were studied. High conversion (78–90 %) in 6 h and full conversion was achieved within 24 h using HPLC to monitor the reaction. Overall, this system is presented as a chemoselective N-debenzylation alternative for aryl-halogenated substrates.
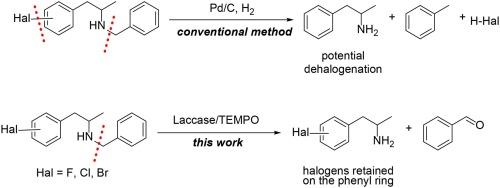
利用漆酶 LAC97/TEMPO 系统对芳基卤化胺进行化学选择性 N-脱苄基氧化反应
该研究报道了利用漆酶 LAC97/TEMPO 系统对芳基卤化胺进行 N-脱苄基反应的情况,结果表明该反应具有选择性的 N-脱苄基作用,而不会出现传统脱苄基方法(如钯催化氢化)中出现的苯环脱卤现象。该反应是在酸性缓冲液中的环境条件下进行的,5% 的二甲基亚砜(DMSO)作为辅助溶剂,在一个露天容器中进行。在含有不同卤素分子的底物上,该系统的多功能性和稳健性得到了验证。此外,还研究了漆酶 LAC97/TEMPO 系统的局限性,强调了在特定底物上由于过度氧化而形成的副产品。研究了一系列含有不同卤素的底物。使用 HPLC 监控反应,6 小时内实现了高转化率(78-90%),24 小时内实现了完全转化。总之,该系统可作为芳基卤化底物的化学选择性 N-脱苄基反应的替代方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


