Multimodal three-dimensional characterization of murine skeletal muscle micro-scale elasticity, structure, and composition: Impact of dysferlinopathy, Duchenne muscular dystrophy, and age on three hind-limb muscles
IF 3.3
2区 医学
Q2 ENGINEERING, BIOMEDICAL
Journal of the Mechanical Behavior of Biomedical Materials
Pub Date : 2024-09-17
DOI:10.1016/j.jmbbm.2024.106751
引用次数: 0
Abstract
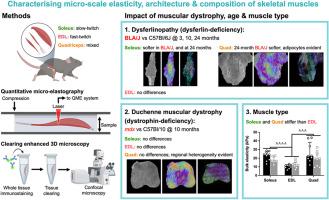
Skeletal muscle tissue function is governed by the mechanical properties and organization of its components, including myofibers, extracellular matrix, and adipose tissue, which can be modified by the onset and progression of many disorders. This study used a novel combination of quantitative micro-elastography and clearing-enhanced three-dimensional (3D) microscopy to assess 3D micro-scale elasticity and micro-architecture of muscles from two muscular dystrophies: dysferlinopathy and Duchenne muscular dystrophy, using male BLA/J and mdx mice, respectively, and their wild-type (WT) controls. We examined three muscles with varying proportions of slow- and fast-twitch myofibers: the soleus (predominantly slow), extensor digitorum longus (EDL; fast), and quadriceps (mixed), from BLA/J and WTBLA/J mice aged 3, 10, and 24 months, and mdx and WTmdx mice aged 10 months. Both dysferlin deficiency and age reduced the elasticity and variability of elasticity of the soleus and quadriceps, but not EDL. Overall, the BLA/J soleus was 20% softer than WT and less mechanically heterogeneous (−14% in standard deviation of elasticity). The BLA/J quadriceps at 24 months was 72% softer than WT and less mechanically heterogeneous (−59% in standard deviation), with substantial adipose tissue accumulation. While mdx muscles did not differ quantitatively from WT, regional heterogeneity was evident in micro-scale elasticity and micro-architecture of quadriceps (e.g., 11.2 kPa in a region with marked pathology vs 3.8 kPa in a less affected area). These results demonstrate differing biomechanical changes in hind-limb muscles of two distinct muscular dystrophies, emphasizing the potential for this novel multimodal technique to identify important differences between various myopathies.
小鼠骨骼肌微尺度弹性、结构和组成的多模态三维表征:铁蛋白沉积症、杜氏肌营养不良症和年龄对三块后肢肌肉的影响
骨骼肌组织的功能受其组成部分(包括肌纤维、细胞外基质和脂肪组织)的机械特性和组织结构的影响,而这些特性和组织结构会因多种疾病的发生和发展而改变。本研究采用定量微弹性成像和透明增强三维(3D)显微镜的新颖组合,以雄性BLA/J小鼠和mdx小鼠及其野生型(WT)对照组为对象,分别评估了两种肌肉萎缩症(钝铁病和杜氏肌肉萎缩症)肌肉的三维微尺度弹性和微结构。我们研究了三块肌肉,它们的慢速肌纤维和快速肌纤维的比例各不相同:比目鱼肌(主要是慢速肌)、伸肌(EDL;快速肌)和股四头肌(混合肌),分别来自 3 个月、10 个月和 24 个月大的 BLA/J 和 WTBLA/J 小鼠,以及 10 个月大的 mdx 和 WTmdx 小鼠。dysferlin 缺乏和年龄都会降低比目鱼肌和股四头肌的弹性和弹性变异性,但不会降低 EDL。总体而言,BLA/J比目鱼肌比WT柔软20%,机械异质性较低(弹性标准偏差-14%)。24 个月时,BLA/J 股四头肌比 WT 软 72%,机械异质性较低(标准偏差-59%),并有大量脂肪组织堆积。虽然 mdx 肌肉与 WT 肌肉在数量上没有差异,但在股四头肌的微尺度弹性和微结构上却存在明显的区域异质性(例如,病变明显的区域为 11.2 kPa,而病变较轻的区域为 3.8 kPa)。这些结果表明,两种不同肌肉萎缩症的后肢肌肉发生了不同的生物力学变化,强调了这种新型多模态技术在识别各种肌病之间重要差异方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of the Mechanical Behavior of Biomedical Materials
工程技术-材料科学:生物材料
CiteScore
7.20
自引率
7.70%
发文量
505
审稿时长
46 days
期刊介绍:
The Journal of the Mechanical Behavior of Biomedical Materials is concerned with the mechanical deformation, damage and failure under applied forces, of biological material (at the tissue, cellular and molecular levels) and of biomaterials, i.e. those materials which are designed to mimic or replace biological materials.
The primary focus of the journal is the synthesis of materials science, biology, and medical and dental science. Reports of fundamental scientific investigations are welcome, as are articles concerned with the practical application of materials in medical devices. Both experimental and theoretical work is of interest; theoretical papers will normally include comparison of predictions with experimental data, though we recognize that this may not always be appropriate. The journal also publishes technical notes concerned with emerging experimental or theoretical techniques, letters to the editor and, by invitation, review articles and papers describing existing techniques for the benefit of an interdisciplinary readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


