Low-energy electric shock ameliorates cell proliferation, morphallaxis, and regeneration via driving key regenerative proteins in earthworm and 3T3 cells
IF 4.8
2区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Electric stimulation regulates many cellular processes like cell proliferation, differentiation, apoptosis and cellular migration. Despite its crucial role in regulating stem cells and regeneration, it remains underexplored in both in-vivo and in-vitro settings. In this study, Eudrilus eugeniae are subjected to electric stimulation (1.5 V) prior and after amputation and which augments regeneration up to double-time. Blocking epimorphosis using 2 M thymidine retracts regeneration kinetics to one-third but such inhibition was rescued by applying electric stimulation which propels an overactive morphallaxis pattern of regeneration. Excreting electric stimulation on control worms shows minimal impact, whereas it enhances the key regenerative proteins like VEGF, COX2, YAP, c-Myc, and Wnt3a on amputated worms. Upon blocking epimorphosis, all these key regenerative proteins are down-regulated but through electric stimulation, the cells are reprogrammed to express a triple fold of the mentioned regenerative proteins, that further promotes morphallaxis. In 3T3 cells, electric stimulation accelerates cell proliferation and migrations in 5 secs exposure and it exerts its function by overexpressing VEGF mediated by MEK1. Wnt3a expression was gradually upregulated in increasing exposure (5 and 25 secs) which aids in maintaining the stemness property. The molecular mechanism underlying regeneration capability can assist in designing novel therapeutic applications.
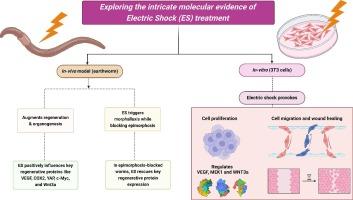
低能量电击通过驱动蚯蚓和 3T3 细胞中的关键再生蛋白,改善细胞增殖、变形和再生能力
电刺激可调节许多细胞过程,如细胞增殖、分化、凋亡和细胞迁移。尽管电刺激在调节干细胞和再生方面起着至关重要的作用,但在体内和体外环境中对它的探索仍然不足。在这项研究中,对截肢前后的乌贼进行电刺激(1.5 V),可使其再生能力提高一倍。使用 2 M 胸苷阻断外蜕变可使再生动力学减慢至三分之一,但施加电刺激后,这种抑制作用得以缓解,因为电刺激可推动过度活跃的形态再生模式。电刺激对对照组蠕虫的影响微乎其微,而对截肢蠕虫则会增强关键再生蛋白,如血管内皮生长因子、COX2、YAP、c-Myc 和 Wnt3a。在阻断外生畸形时,所有这些关键再生蛋白都会下调,但通过电刺激,细胞会重新编程,表达上述再生蛋白的三倍,从而进一步促进变形。在 3T3 细胞中,电刺激可在 5 秒钟的暴露时间内加速细胞增殖和迁移,并通过 MEK1 介导的血管内皮生长因子的过度表达发挥其功能。Wnt3a的表达随着暴露时间(5秒和25秒)的增加而逐渐上调,这有助于维持干性特性。再生能力的分子机制有助于设计新的治疗应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioelectrochemistry
生物-电化学
CiteScore
9.10
自引率
6.00%
发文量
238
审稿时长
38 days
期刊介绍:
An International Journal Devoted to Electrochemical Aspects of Biology and Biological Aspects of Electrochemistry
Bioelectrochemistry is an international journal devoted to electrochemical principles in biology and biological aspects of electrochemistry. It publishes experimental and theoretical papers dealing with the electrochemical aspects of:
• Electrified interfaces (electric double layers, adsorption, electron transfer, protein electrochemistry, basic principles of biosensors, biosensor interfaces and bio-nanosensor design and construction.
• Electric and magnetic field effects (field-dependent processes, field interactions with molecules, intramolecular field effects, sensory systems for electric and magnetic fields, molecular and cellular mechanisms)
• Bioenergetics and signal transduction (energy conversion, photosynthetic and visual membranes)
• Biomembranes and model membranes (thermodynamics and mechanics, membrane transport, electroporation, fusion and insertion)
• Electrochemical applications in medicine and biotechnology (drug delivery and gene transfer to cells and tissues, iontophoresis, skin electroporation, injury and repair).
• Organization and use of arrays in-vitro and in-vivo, including as part of feedback control.
• Electrochemical interrogation of biofilms as generated by microorganisms and tissue reaction associated with medical implants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


