Bimetallic complexation for significant fluorescence enhancement of faecal pigment towards water quality testing
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-09-21
DOI:10.1016/j.jphotochem.2024.116041
引用次数: 0
Abstract
Systematic spectroscopic investigations revealed bimetallic complexation of faecal pigments (FP) in water through their multiple binding sites. FP-Zn(II)/Gd(III) complexes showed narrow emission (FWHM ∼ 26 nm) with maximum intensity compared to different bimetallic complexes in water. The fluorescence intensity of bimetallic Zn(II)/Gd(III) complexes of FP increased ∼3–5 fold in aqueous media compared to the FP-Zn(II) complexes, i.e. well-known as Schlesinger’s test. The optimum Zn(II): Gd(III) stoichiometry was observed at 3:2 for maximum FP fluorescence enhancement. The bimetallic FP-Zn(II)/Gd(III) complexes also exhibited higher fluorescence lifetimes and quantum yield than FP-Zn(II) complexes. The photophysical investigations demonstrated that bimetallic Zn(II)/Gd(III) complexation increased the rigidity of chromophores present in FP. Consequently, a decrease in non-radiative decay rate constants (knr) by ∼ 1.5 times was observed for bimetallic complexes compared to FP-Zn(II) in water. Hence, a detection limit of the nanomolar concentration range of FP could be achieved in the aqueous media. The effect of diverse interfering factors (humic acid, water hardness, pH) on the fluorescence behaviour of FP-Zn(II)/Gd(III) complexes was also investigated with an approach mimicking real water samples. Finally, a simple non-extraction analytical approach for sensitive detection of FP in water was demonstrated while also considering the fluorescence matrix interference problem.
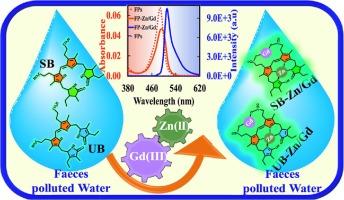
双金属络合可显著增强粪便色素的荧光,用于水质检测
系统光谱研究发现,粪便色素(FP)通过其多个结合位点在水中形成双金属络合物。与不同的双金属络合物相比,FP-Zn(II)/Gd(III)络合物在水中显示出窄幅发射(FWHM ∼ 26 nm)和最大强度。与 FP-Zn(II) 复合物相比,FP 的 Zn(II)/Gd(III) 双金属复合物在水介质中的荧光强度增加了 3-5 倍,即著名的施莱辛格试验。最佳的 Zn(II):Gd(III)的最佳配比为 3:2,可最大程度地增强 FP 的荧光。双金属 FP-Zn(II)/Gd(III) 复合物的荧光寿命和量子产率也高于 FP-Zn(II)复合物。光物理研究表明,双金属 Zn(II)/Gd(III) 复合物增加了 FP 中发色团的刚性。因此,与水中的 FP-Zn(II)相比,双金属络合物的非辐射衰变速率常数(knr)降低了 1.5 倍。因此,水介质中 FP 的检测限可达到纳摩尔浓度范围。此外,还采用模拟真实水样的方法研究了各种干扰因素(腐植酸、水硬度、pH 值)对 FP-Zn(II)/Gd(III) 复合物荧光行为的影响。最后,在考虑荧光基质干扰问题的同时,还展示了一种用于灵敏检测水中 FP 的简单非萃取分析方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


