Tuning the sulfide interface of MnCo2O4-based nanostructures enables efficient water/seawater electrolysis
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Hydrogen generation through water electrolysis is greatly dependent on the development of energy and time-efficient techniques to construct stable and active electrocatalysts for oxygen evolution reaction (OER). Currently, major research focuses on producing hydrogen through direct seawater electrolysis instead of fresh water to build a sustainable society. However, competitive reactions such as chlorine evolution reaction (CIER) beyond OER and electrode erosion issues make seawater electrolysis more difficult. Here, we report an interfacial engineering strategy that constructs a MnCo2O4@CoS hybrid structure by sulfurization of the spinal MnCo2O4 nanowires. The hybrid structure demonstrates an excellent OER performance in electrolytes containing alkaline and saltwater. Specifically, the prepared catalyst needs overpotentials of 205 mV and 225 mV to deliver a current density of 10 mA cm−2 in 1 M KOH and alkaline seawater when used as OER electrocatalysts. This should be noted that the CoS layer on the surface of MnCo2O4 nanowires not only acts as a Cl‾ protective layer to impede electrode erosion and CIER but also provides metallic ions with a higher valence state to enhance the intrinsic catalytic activity of water oxidization. Thus, this type of electrocatalyst could represent a favorable choice, carrying substantial implications for hydrogen-based economies and environmental enhancement.
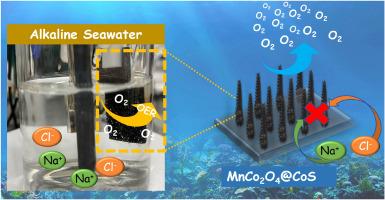
调整基于 MnCo2O4 的纳米结构的硫化物界面可实现高效水/海水电解
通过电解水制氢在很大程度上依赖于开发节能省时的技术,以构建稳定而活跃的氧进化反应(OER)电催化剂。目前,主要的研究重点是通过直接电解海水而不是淡水来制氢,以建设一个可持续发展的社会。然而,OER 之外的氯进化反应(CIER)等竞争反应以及电极侵蚀问题使得海水电解更加困难。在此,我们报告了一种界面工程策略,即通过硫化 MnCo2O4 纳米线来构建 MnCo2O4@CoS 混合结构。该杂化结构在含有碱性和盐水的电解质中表现出优异的 OER 性能。具体来说,所制备的催化剂在 1 M KOH 和碱性海水中用作 OER 电催化剂时,需要 205 mV 和 225 mV 的过电位才能提供 10 mA cm-2 的电流密度。值得注意的是,MnCo2O4 纳米线表面的 CoS 层不仅可以作为 Cl‾ 保护层,阻碍电极侵蚀和 CIER,还能提供价态更高的金属离子,提高水氧化的内在催化活性。因此,这种类型的电催化剂可能是一种有利的选择,对基于氢的经济和环境改善具有重大意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


