Gene hunting and semi-rational design of carbonyl reductase from Kosakonia radicincitans for highly efficient synthesis of the key chiral intermediate of Telotristat ethyl
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Carbonyl reductase exhibits significant potential in the asymmetric production of chiral alcohols. (αR)-4-Chloro-2-(3-methyl-1H-pyrazol-1-yl)-α-(trifluoromethyl)benzenemethanol ((R)-CMPPFO) is a critical precursor for the synthesis of Telotristat ethyl, an oral drug for the treatment of diarrhea in carcinoid syndrome. Herein, a novel carbonyl reductase KrSDR5 from Kosakonia radicincitans was obtained using gene hunting strategy, capable of asymmetrically reducing the precursor ketone 1-[4‑chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl]-2,2,2-trifluoroethanone (CMPPFA) to (R)-CMPPFO with strict R-stereoselectivity (>99.9 % ee). Further, semi-rational design was adopted to acquire a positive mutant KrSDR5T91V/V141M/I159V, with assistance from a comparative analysis of enzyme-substrate binding mode in molecular dynamics (MD) simulations. This variant displayed a 12.4-fold increase in kcat/Km towards CMPPFA compared to the wild-type (WT) KrSDR5. Insights were gained on the high enantioselectivity and the enhancement of enzyme catalytic activity of the mutant through MD simulations. Using the whole-cells of KrSDR5T91V/V141M/I159V as biocatalyst, the asymmetric synthesis of (R)-CMPPFO was achieved within 20 h at 500 mM CMPPFA concentration, resulting in a 95.0 % yield with >99.9 % ee, and a highest space-time yield (STY) of 165.7 g·L-1·d-1 compared with previous reports. This study provides a robust biocatalyst for highly efficient production of the key precursor (R)-CMPPFO for Telotristat ethyl, highlighting its potential in the biosynthesis of pharmaceutical intermediates.
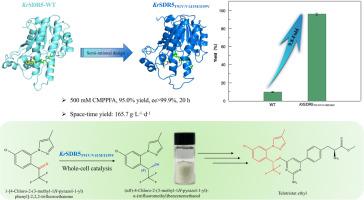
从 Kosakonia radicincitans 中获取基因并对羰基还原酶进行半合理设计,以高效合成泰罗司他乙酯的关键手性中间体
羰基还原酶在不对称生产手性醇方面具有巨大潜力。(αR)-4-氯-2-(3-甲基-1H-吡唑-1-基)-α-(三氟甲基)苯甲醇((R)-CMPPFO)是合成治疗类癌综合征腹泻的口服药物泰罗司他乙酯的重要前体。本文采用基因猎取策略,从Kosakonia radicincitans中获得了一种新型羰基还原酶KrSDR5,它能够将前体酮1-[4-氯-2-(3-甲基-1H-吡唑-1-基)苯基]-2,2,2-三氟乙酮(CMPPFA)不对称地还原为(R)-CMPPFO,并具有严格的R-严格选择性(>99.9 % ee)。此外,在分子动力学(MD)模拟中对酶与底物结合模式的比较分析的帮助下,采用半合理设计获得了正突变体 KrSDR5T91V/V141M/I159V。与野生型(WT)KrSDR5 相比,该变体对 CMPPFA 的 kcat/Km 增加了 12.4 倍。通过 MD 模拟,我们对突变体的高对映选择性和酶催化活性的增强有了更深入的了解。以 KrSDR5T91V/V141M/I159V 的全细胞作为生物催化剂,在 500 mM CMPPFA 浓度下,20 h 内实现了 (R)-CMPPFO 的不对称合成,与之前的报道相比,产率达 95.0 %,ee 为 99.9 %,最高时空产率(STY)为 165.7 g-L-1-d-1。这项研究为高效生产泰洛司他乙酯的关键前体 (R)-CMPPFO 提供了一种稳健的生物催化剂,凸显了其在医药中间体生物合成方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


