Sm3+ activated Ba3LaNa(PO4)3F fluorophosphate phosphor: Synthesis, characterization and their photoluminescence investigation for warm WLEDs
IF 3.8
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
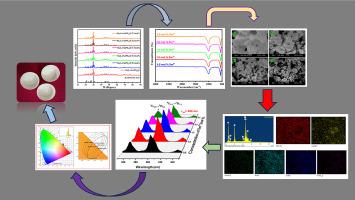
In this present research report, the series of Ba3LaNa(PO4)3Fphosphor doped with Sm3+ was successfully synthesized by using a high-temperature solid-state reaction method. The phase purity was examined using X-ray diffraction (XRD), which confirmed a pure phase of prepared material after sintering the sample at 700 °C. Fourier transform infrared (FT-IR) spectra showed absorption bands corresponding to the stretching and vibrational modes of PO4 in phosphate. Scanning electron microscopy (SEM) images displayed the morphology of sample and agglomerated structure of micron-sized particles. Photoluminescence excitation (PLE) and emission spectra of the Ba3LaNa(PO4)3F: Sm3+ phosphors were studied. When excited with near-UV light, the phosphor showed three prominent peaks at 567, 604, and 643 nm. The emission spectra for different concentrations of Sm3+ ions were also measured to find the optimal dopant concentration. The highest intensity was observed at a 2.0 mol% concentration of Sm3+ ions at 604 nmwith concentration quenching occurring beyond this point. The CIE chromaticity coordinates for the different concentration phosphor were located in the orange-red region, indicating its characteristic light emission. So therefore, these results suggested that the synthesized reddish-orange light-emitting Ba3LaNa(PO4)3F: Sm3+prepared phosphor is promising for applications in white light-emitting diodes (WLEDs).
Sm3+ 活化的 Ba3LaNa(PO4)3F 磷酸盐荧光粉:用于暖色 WLED 的合成、表征及其光致发光研究
本研究报告采用高温固态反应方法,成功合成了掺杂 Sm3+ 的 Ba3LaNa(PO4)3F 磷酸盐系列。利用 X 射线衍射 (XRD) 对相纯度进行了检测,结果表明在 700 °C 下烧结样品后,所制备材料的相为纯相。傅立叶变换红外光谱(FT-IR)显示了与磷酸盐中 PO4 的伸展和振动模式相对应的吸收带。扫描电子显微镜(SEM)图像显示了样品的形态和微米级颗粒的团聚结构。研究了 Ba3LaNa(PO4)3F:Sm3+ 磷酸盐的光致发光激发(PLE)和发射光谱。当用近紫外光激发时,荧光粉在 567、604 和 643 纳米波长处显示出三个显著的峰值。为了找到最佳掺杂浓度,还测量了不同浓度 Sm3+ 离子的发射光谱。浓度为 2.0 mol% 的 Sm3+ 离子在 604 nm 处的发射光谱强度最高,超过该点时会出现浓度淬灭。不同浓度荧光粉的 CIE 色度坐标均位于橙红区域,表明其发光特性。因此,这些结果表明合成的橘红色发光 Ba3LaNa(PO4)3F:Sm3+ 制备的荧光粉有望应用于白光发光二极管(WLED)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


