Simultaneous adsorption of cadmium, zinc, and lead ions from aqueous solution by Montmorillonite clay coated with MgCuAl-LDH nanoparticles
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this work, Montmorillonite (MMt) coated with MgCuAl-layered double hydroxide (LDH) nanoparticles as a new adsorbent (MCA-LDH@MMt) was synthesized via a low supersaturation characterized using BET, TEM, XRD, SEM/EDS, and FT-IR analysis. The adsorption studies were conducted in a ternary-batch system of three heavy metal ions (cadmium, zinc, and lead). The results showed that the MMt was successfully loaded with MgCuAl nanoparticles with a porosity of 44.63 % and a specific surface area of 76.63 m2/g. In addition, the surface morphology analysis showed that there were several changes in elemental dispersion, molar ratio, and molecular weights during the preparation of the used adsorbent. The influence of environmental parameters on the adsorption behavior was studied in detail, whereby the maximum adsorption capacity for the three metals ions was achieved at pH 5, 120 min contact time, 0.2 g/100 mL dose, and 50 mg/l initial metal ion concentration at 25 ± 1 °C. A pseudo-second-order model well correlates the kinetic data of the three metal ions (R2 > 0.991). The Cd2+ and Zn2+ isotherm data exhibited high compatibility with the Langmuir model, while the Freundlich model better fitted the Pb2+ isotherm data. The maximum adsorption capacity from the Langmuir model was 91.6, 164.9, and 129.2 mg/g for Cd2+, Zn2+, and Pb2+, respectively. Also, the adsorption process of the three metal ions onto was primarily characterized by their spontaneous and exothermic nature. In conclusion, this study demonstrated that MCA-LDH@MMt is an effective adsorbent for the simultaneous adsorption of cadmium, zinc, and lead in aqueous solution, with the ability to recover the synthesized adsorbent after four consecutive cycles with a minimal reduction in adsorption ability.
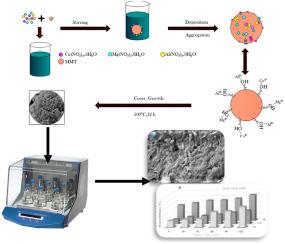
涂有 MgCuAl-LDH 纳米颗粒的蒙脱石粘土同时吸附水溶液中的镉、锌和铅离子
本研究通过低过饱和合成了蒙脱石(Montmorillonite,MMt),并使用 BET、TEM、XRD、SEM/EDS 和 FT-IR 分析对其进行了表征,将其涂上 MgCuAl 层状双氢氧化物(LDH)纳米颗粒作为一种新型吸附剂(MCA-LDH@MMt)。在三种重金属离子(镉、锌和铅)的三元批处理体系中进行了吸附研究。结果表明,MMt 成功负载了 MgCuAl 纳米颗粒,其孔隙率为 44.63%,比表面积为 76.63 m2/g。此外,表面形貌分析表明,在制备所用吸附剂的过程中,元素分散度、摩尔比和分子量发生了一些变化。详细研究了环境参数对吸附行为的影响,在 pH 值为 5、接触时间为 120 分钟、剂量为 0.2 克/100 毫升、初始金属离子浓度为 50 毫克/升、温度为 25 ± 1 °C的条件下,三种金属离子的吸附容量达到最大。伪二阶模型很好地关联了三种金属离子的动力学数据(R2 > 0.991)。Cd2+ 和 Zn2+ 等温线数据与 Langmuir 模型具有很高的兼容性,而 Freundlich 模型则更好地拟合了 Pb2+ 等温线数据。根据 Langmuir 模型,Cd2+、Zn2+ 和 Pb2+ 的最大吸附容量分别为 91.6、164.9 和 129.2 mg/g。此外,三种金属离子在 MCA-LDH@MMt 上的吸附过程主要表现为自发和放热。总之,本研究证明 MCA-LDH@MMt 是一种有效的吸附剂,可同时吸附水溶液中的镉、锌和铅,并能在连续四次循环后回收合成的吸附剂,且吸附能力下降很小。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


