Interrogation of three-finger toxin and phospholipase A2 higher order structures from the forest cobra (Naja melanoleuca) venom using a mass spectrometric approach
IF 1.7
3区 化学
Q3 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
Abstract
Snake venoms are composed of bioactive proteins and peptides from various toxin families and elicit potent pharmacological activity. There is great interest in characterising these venom proteins for the development of effective antivenom treatment as well as utilisation for biomedical and therapeutic applications. However, a thorough structural understanding of the snake venom proteins is necessary. Higher-order protein complexes are known to form in snake venoms and lend structural and functional diversity, often eliciting greater activity than the sum of monomeric protein species. Despite the significance, the nature of these protein complexes is extremely underexplored. In this study, we demonstrate the use of mass spectrometry (MS)-based strategies to explore the toxins at a quaternary level in the venom from the medically significant forest cobra (Naja melanoleuca). Small toxins, mainly three finger toxins (3FTxs) and phospholipase A2s (PLA2s), were identified by comparison of intact and chemically reduced masses using matrix-assisted laser desorption ionisation (MALDI-MS) profiling. Notably, interrogation of these small toxins by native MS and collision-induced dissociation revealed the presence of various non-covalent 3FTx and PLA2 dimers, providing insight on the higher-order protein structures for a variety of N. melanoleuca toxins using a MS-based approach. Furthermore, phospholipid substrate specificity of N. melanoleuca PLA2 enzymes were explored, capturing the indiscriminate activity of these PLA2s towards a range of phospholipid classes for the first time.
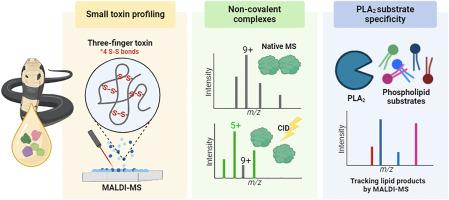
利用质谱方法研究森林眼镜蛇(Naja melanoleuca)毒液中的三指毒素和磷脂酶 A2 的高阶结构
蛇毒由来自不同毒素家族的生物活性蛋白质和肽组成,具有强大的药理活性。为了开发有效的抗蛇毒血清治疗方法,以及将蛇毒应用于生物医学和治疗领域,人们对研究这些蛇毒蛋白的特征有着浓厚的兴趣。然而,彻底了解蛇毒蛋白的结构是必要的。众所周知,蛇毒中会形成高阶蛋白复合物,这种复合物具有结构和功能多样性,通常比单体蛋白的总和具有更强的活性。尽管意义重大,但这些蛋白质复合物的性质却极少被探索。在这项研究中,我们展示了如何利用基于质谱的策略来探索具有重要医学价值的森林眼镜蛇(Naja melanoleuca)毒液中的四级毒素。利用基质辅助激光解吸电离(MALDI-MS)分析法,通过比较完整质量和化学还原质量,确定了小毒素,主要是三指毒素(3FTxs)和磷脂酶 A2s(PLA2s)。值得注意的是,通过本征质谱和碰撞诱导解离对这些小毒素进行检测,发现了各种非共价的 3FTx 和 PLA2 二聚体的存在,从而利用基于质谱的方法深入了解了多种 N. melanoleuca 毒素的高阶蛋白质结构。此外,研究人员还探索了 N. melanoleuca PLA2 酶的磷脂底物特异性,首次捕获了这些 PLA2 对一系列磷脂类别的无差别活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.60
自引率
5.60%
发文量
145
审稿时长
71 days
期刊介绍:
The journal invites papers that advance the field of mass spectrometry by exploring fundamental aspects of ion processes using both the experimental and theoretical approaches, developing new instrumentation and experimental strategies for chemical analysis using mass spectrometry, developing new computational strategies for data interpretation and integration, reporting new applications of mass spectrometry and hyphenated techniques in biology, chemistry, geology, and physics.
Papers, in which standard mass spectrometry techniques are used for analysis will not be considered.
IJMS publishes full-length articles, short communications, reviews, and feature articles including young scientist features.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


